
連絡先:
携帯 090-8257-9756
メール masahiko602@gmail.com
LINE masahiko_okada
全体が5つのページに分割されています。各ページの
最後にある「以前のページへ」という文字をクリックしていくと、
すべての記事を掲載日をさかのぼって閲覧することができます。
先週1週間の総アクセス回数: 4,609
テレビでは語られない世界の最新情報を独自に分析
正しい情報を偏りなく
このページの最後に表示してある 目 次 から以前の主な記事を閲覧する
ことができます(青文字をクリック)
「ワクチンの真実とやさしい解説」はこちらの動画でどうぞ!
スマホで閲覧する場合→

(この動画は2021年2月19日に投稿したものですが、2022年7月、
突然、削除されてしまいました。その動画をそのまま米国の動画
サイトに再投稿したものが本編です。情報が何もなかった時期に
作成したものですから、不正確な表現も何か所かあります。それも
歴史の一コマとして、そのまま掲載してあります。正しい情報は
当ホームページでご確認ください)
English version is available here!
改造メッセンジャーRNAの専門的解説はこちらでどうぞ!
今週の新情報
(2026.3.2)
Q&A インフルエンザ・ワクチン再考?
『中間まとめ第3回』で、インフルエンザ・ワクチンの問題点を記しましたが、その後、新たなデータがいくつか発表されました。そこで今回は、新型コロナワクチンの話題から少し離れ、インフルエンザ・ワクチンについての最新情報をまとめます。
インフルエンザのワクチンは、前年の流行株をもとに鶏卵を用いて作られますので、毎年、その中身(抗原)が変わっていきます。2025~2026年のシーズンに向けては、流行の主流となっているA型のH3N2と、それに加えてA型のH1N1(pdm09)、B型のビクトリア系統の3つを含むワクチンが使われています。HやNはインフルエンザ・ウイルスの表面にある突起物のことで、スパイク蛋白と異なり、それ自体に毒性はありません。
2025年の暮れ、米国の研究者グループは、このインフルエンザ・ワクチンの効果について大規模な調査を行い、結果を発表しました(文献1)。対象となったのは米国の巨大な非営利医療機関グループの職員43,920人で、毎年、インフルエンザ・ワクチンの接種を義務として受けることになっています。調査期間は2024年10月1日から2025年5月21日までです。
調査の目的は、「ワクチン接種を受ける前の時期」と、「受けたあとの時期」におけるインフルエンザ感染の割合を比べることでした。職員数が多いため、接種を早い時期に受けた人がいれば、調査の終わりのほうで受けた人もいて、時期が分散していたために、このような分け方が可能でした。
240日に及ぶ追跡期間中、「ワクチンをすでに打った人たち」と「ワクチンをまだ打っていない人たち」の感染率を比べたところ、以下のような結果となりました(グラフは、論文で提示されている図をもとに私がイラストにしたもの)。
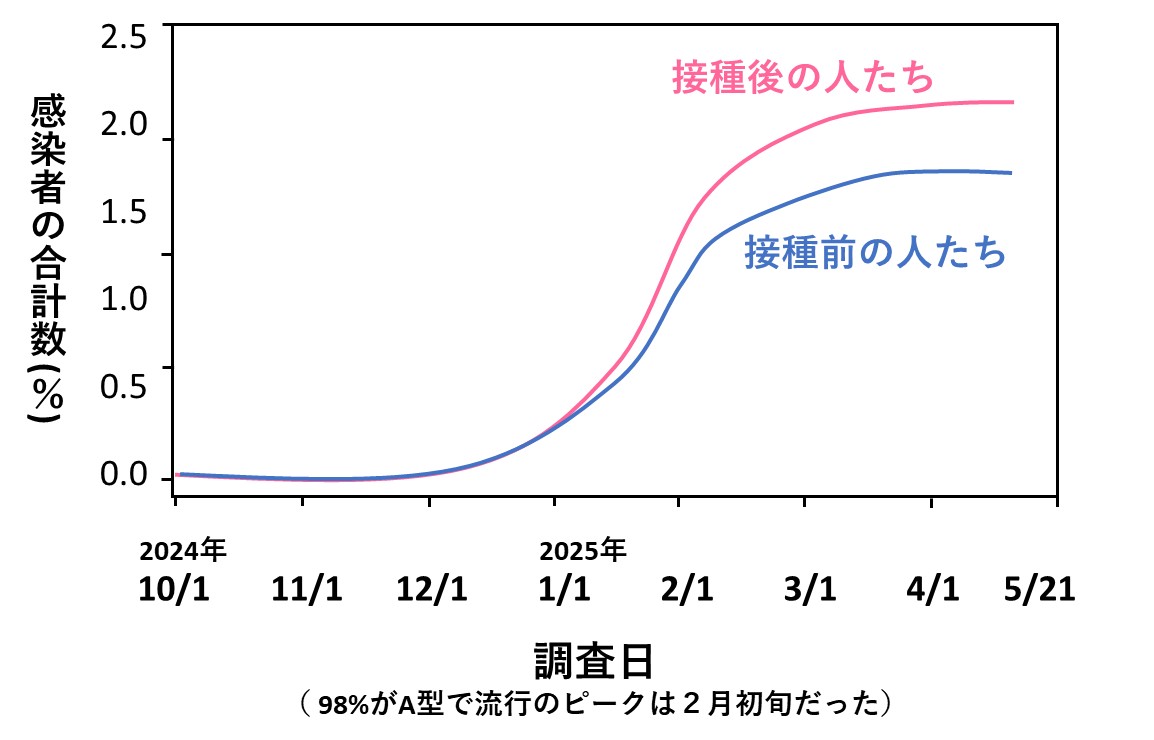
2つのグループの感染率は徐々に離れていき、3月下旬ころには、統計学的に有意とされるほどの差になっていました。もちろん年齢や性別、医療機関での職種や勤務地などで感染のしやすさに違いが生じる可能性がありますから、多変量調整という方法で、これらの影響は消去されました。
2つのグループの間には、ほかにも何か隠れた背景があって、結果に影響を与えていた可能性もあります。そこでE-valueと呼ばれる最新の計算法(文献2)を駆使し、そのような問題はないことも証明しています。さらに、PCR検査の結果が陽性だった割合も、両グループで差がありませんでした。
以上のことから、この論文の信頼度は非常に高いと判断できます。
実は、同じ時期にインフルエンザ・ワクチンの有効率を調べた研究がもうひとつあり、「同ワクチンは有効」との結論を報告していました(文献3)。しかし、信頼性に劣る「テスト陰性調査」(中間まとめ第6回を参照)で行なわれたものであったため、結果は当てになりません。
ワクチンを打った人たちのほうで感染率が高くなっていた理由は、すでに『中間まとめ第3回』で記した抗原原罪理論に従った免疫反応が起こるからです。この理論が成立するのは、年々、少しずつ変異するようなウイルスに対するワクチンの場合に限られますが、インフルエンザ・ウイルスがまさにそれなのです。
「mRNAワクチンは危険だと思うけど、ワクチンをすべてを否定しているわけではない・・・」とのご意見をよく耳にします。しかし、新型コロナワクチンの騒動を通じて、「ワクチン」そのものの理論やメカニズムに対する理解が大きく変わってきました。発想の転換が必要かもしれません。
【参考文献】
1) Shrestha NK, et al., Effectiveness of the influenza vaccine during the 2024-2025 respiratory viral season: a prospective cohort study. medRxiv, Oct 11, 2025.
2) VanderWeele TJ, et al., Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med, Aug 17, 2017.
3) Frutos AM, et al., Interim estimates of 2024-2025 seasonal influenza vaccine effectiveness - four vacine effectiveness networks, United States, October 2024-February 2025. MMWR, Feb 27, 2025.
先週までの情報
(2026.2.23)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第22回)?
新型コロナワクチンによる健康被害について、エビデンスの中間まとめを行ってきました。前回は、その総合討論として、責任がもっとも重いのは「製薬企業」であり、次いで「国が主催する各種委員会・分科会の委員長や座長」であることの根拠をまとめました。今回はその続きです。
No.3 官僚と政治家の責任を問う
国の委員会や分科会の委員として選ばれた人は、すべてワクチンを推進する立場の人であったことから、人選に著しい偏りがあったことになります。その人選を行ったのが誰なのかを確認できれば、官僚と政治家の意図がわかることになりそうです。
いずれにしろ、国の委員会や分科会を主導した官僚の責任は重大です。薬害エイズを巡る議論では、「世界中の情報をほぼリアルタイムで取得することができていたのは(旧)厚労省であり、結果的には、官僚主導のリーダーシップ以外、当時の状況を打開する術はなかった」(文献1)との分析もありました。この状況は、新型コロナワクチンでも同じです。
ただし、「担当の官僚が、適切な方法で危険情報について上司に報告し、相談していたとしたら、当人にだけ責任を問うことはできない」(文献2)との見解も一方で呈されていましたので、その証明は必ずしも簡単でないかもしれません。
すでに『中間まとめ』で述べたように、上司を辿っていけば、行きつき先は厚生労働大臣、ワクチン担当大臣であり、内閣総理大臣です。一般論で言えば、政治家に医学などの専門知識はありませんから、官僚の報告を信ずるしかありません。たとえリスクについての報告があったとしても、表現の仕方によっては重大事との認識をもてなかったかもしれません。
逆に、突然の感染症大流行に怯えた政治家が、官僚に対して上位下伝で「とにかくワクチンを早く」と指示した可能性もあります。
どちらにしろ、両者のどちらかに重大責任があったことに間違いはありません。したがって、裁判を起こす目的のひとつは、官僚と政治家がどのように関与していたのか、その背景を明らかにすることです。
No.4 製薬企業の広告に登場した大学教授
イタリアの地震騒動では、間違った判断をした地震学者が告訴されましたが、学術的な意見を会議で述べただけであったという理由で最終的に無罪となりました。一方、担当官僚の1人がテレビで誤った地震情報を流したとして有罪になった、という出来事を『中間まとめ』で記しました。
新型コロナワクチンについては、多くの医師や研究者がテレビでワクチンの接種を推奨し、リスクについての言及をまったくしませんでした。とくに気になったのは旧国立大学の某教授が、接種を促したいワクチンメーカーの新聞広告に登場していたことです。
すでに『中間まとめ』で記したように、旧国立大学の職員は「みなし公務員」です。教授などが本来の業務以外の仕事をして報酬を得る場合は、国家公務員倫理法により『兼業届』、あるいは『兼業許可申請書』という書類を大学長あてに提出することになっています。そこには、いつ、どこで、何をして、いくらの報酬を得るのかを具体的に書かなければなりません。
通常は、「本業で得た専門的知識で地域に貢献するため」との前提があり、また届け出をすることによって情報を公開したことにもなるため、審査は行われません。しかし、前例のない兼業や利益相反が問題となるような場合は、倫理委員会などで審査が行われます。かつて、私も大学の倫理審査委員会に属していましたが、内容に立ち入った審査を行なったこともありました。
したがって裁判では、本人から兼業届が提出されていたか、されていたとすれば大学はどのような判断をしたのかについて、情報公開を求める必要があるでしょう。
なお、頻回にテレビに出演してワクチン接種を勧めていた医師の多くは、公務員でなく、単に個人的見解を述べていたに過ぎないため、法的な責任を追及するのは難しいかもしれません。
民事裁判は和解で決着することも多く、それでは世論の喚起につながりません。裁判が社会問題の提起につながることを祈って『中間まとめ』をこれで最終回とし、次回からは、従来どおり「確かなエビデンス」の紹介を再開します。
【参考文献】
1) 花井十伍, 薬害の教訓から考える―薬害エイズと血液行政―, 保健医療社会学論集, 27(2), 2017.
2) 高内寿夫, 行政組織内における責任の構造―薬害エイズを素材にしてー, 白鵬法学, Feb 24, 2017.
(2026.2.16)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第21回)?
新型コロナワクチンによる健康被害について、全19回にわたり『中間まとめ』を行ってきました。前回からは、それらの情報を中心に、お寄せいただいたご意見も交えた総合討論としていますが、今回は裁判で訴えるべき相手は誰なのかを具体的にまとめます。
裁判には「民事裁判と「刑事裁判」の2つがあり、目的や手続きが大きく異なっています。民事裁判は、通常、個人が個人に対して起こすもので、たとえば借金を返さない人を訴えるような場合ですが、国の行為、あるいは不作為によって損害を被り、賠償を求める場合(国家賠償請求訴訟)も民事裁判となります。
一方、刑事裁判は、強盗の犯人を訴追して罰するような場合で、裁判を起こすのは検察官に限られています。一般の人が公害や薬害などに対して刑事裁判を起こしたい場合は、まず警察や検察に告訴状などを提出し、受理される必要があります。薬害エイズでは、民事と刑事の両方の裁判が起こされたのは、前回、記したとおりです。
以下、新型コロナワクチン健康被害をもたらし、あるいは放置した人たちの責任の重さに順位をつけてまとめますが、民事裁判と刑事裁判のいずれにとっても必要な論拠となるはずです。
No.1 もっとも責任が重いのは製薬企業
新型コロナのmRNA ワクチンを販売している製薬企業が、人々の信頼を裏切る形で論文を発表していたことから、もっとも責められるべき存在であることは確かです。
同ワクチンを販売している2つの製薬企業は米国内に本社がありますが、同国では、保健福祉省長官の権限で時限の法律が成立していて、2029年12月31日までワクチンを製造販売している企業を訴えることはできません(文献1,2)。
この法律は日本には適用されないはずですが、「製薬企業と日本政府との間で交わされたワクチン購入に関する秘密の契約書」に、同じ趣旨の文言が記載されていると考えられます。
注目すべきは、この法律に但し書きがあり、「故意の不正行為がない限り」とされていることです。故意の不正行為を指摘するのは簡単でないとしても、「人々の信頼を裏切る行為」があったのは、あきらかです。したがって、この点を、まず裁判で訴えていく必要があります。
No.2 国の各種委員会の会長・座長の責任も見逃せない
製薬企業が発表した論文で話題となったことのひとつは、「ワクチンの有効率は95パーセント」と記されていたことでした。
これを受けて、令和3年2月15日に開催された第19回厚生科学審議会予防接種・ワクチン分科会では、事務局(官僚)と委員(専門家)との間で以下のような意見が交わされていました(文献3;わかりやすくするため若干の言葉の置き換えを行なっています)。
事務局: ワクチンの有効率は、新型コロナの感染歴がない人で95%、感染歴が
ある人も含めると94.6%ということでございます。
委 員: 94.6%が感染があった人も混ぜた値だとすると、これも切り上げて95%
とすればよいのではないか・・・。
議事録で見る限り、分科会では有効率に関してこの程度の意見交換しかなされていませんでした。専門家である委員にも正しく理解されないまま、「95%という数字だけが世の中を独り歩きすること」を容認したのです。
また、分科会とは別に「新型コロナウイルス感染症対策アドバイザリー・ボード」という委員会が厚生労働省に置かれていましたが、そのメンバーの1人が、のちにファイザー社の役員に転身したというニュースもあり、不信感はいっそう募ります。
どの会議でも、各委員が自ら情報を精査することもなく、専門家としての役割を果たしていませんでした。少なくとも、そのまとめ役であった分科会長や座長の責任は見逃すことができません。
ある(元)分科会長は、最近のテレビ番組で「感染防止効果はあまりないワクチンです」と語ったという話をすでに『中間まとめ』でも記しましたが、同じ人が当時のテレビ番組で以下のように発言していたのです。
2021/06/08 ANN:「個人の予防はできるが、集団免疫みたいな考え方は早計」
2021/08/25 TBS:「教職員に優先的にワクチンを接種するなど対策強化を求めた」
2021/09/03 日テレ:「ワクチン接種した人の行動制限を緩和する案を検討している」
2022/04/07 日テレ:「ワクチンを打つと自分の健康を守ることになります」
2022/04/09 TBS:「家族などを守るためにも3回目接種を受けるよう・・・」
どの委員からもワクチンの副作用を懸念する発言はなく、「ワクチンありき」で審議が行なわれていたのです。委員の人選に偏りがあったことはあきらかで、選んだ人(官僚?)の責任も合わせて問う必要があります。
次回は、この続きです。
【参考文献】
1) Sigalos M, You can't sue Pfeizer or Moderna if you have severe Covid vaccine side effect. The government likely won't compensate you for damages either. CNBC, Dec 23, 2020.
2) Kevin HJ, The PREP Act and COVID-19, Part 2: The PREP Act Declaration for COVID-19 countermeasures. Congressional Research Service, Mar 1, 2025.
3) 厚生労働省, https://www.mhlw.go.jp/stf/shingi/shingi-kousei_127713.html
4) 発生までの予防接種の対象疾病 報告基準となる症状 その他の反応. PMDA, https://www.pmda.go.jp/files/000267773.pdf
(2026.2.9)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第20回)?
前回は、厚生労働省とその所轄法人(PMDA)に所属する国家公務員に課せられた法律と、新型コロナワクチン健康被害との関係をまとめました。今回は、『中間まとめ』として全19回にわたって集約してきた情報を中心に、当ホームページあてに届いたご意見も交えた総合討論とします。
まず、当ホームページに届いたお便りには、以下のような貴重なご意見がありました。
・薬害エイズの裁判では、官僚の1人が有罪となったことで「一応の決着がついて事件の幕引きができた」、という雰囲気になってしまった。
・議員有志の会で、未接種者の感染者数に関する質問が出た際、厚労省が接種後2週間を未接種者として扱ってカウントしていたことが暴露され、その後、厚労省はデータを出さなくなった(注: ワクチンを接種しないと感染者が増えるというストーリーを厚労省は創りたかったのではないか、という意味)。
・政権をとっている政治家を「先生」と呼び、特に安倍政権以降、その先生のために法律を犯す官僚が出てくるようになった。また国家公務員である検察や裁判官も、国民より「先生」のほうに忖度し、保身を考えている。
・薬がどのように効くのか知らないで処方している医師もいる。患者も処方された薬を調べないと怖い、と思った。
以上のご意見を踏まえて、論点を整理するため、新型コロナワクチン健康被害の問題を、前回紹介した薬害エイズと比べてみることにします。以下は、その対照表です。
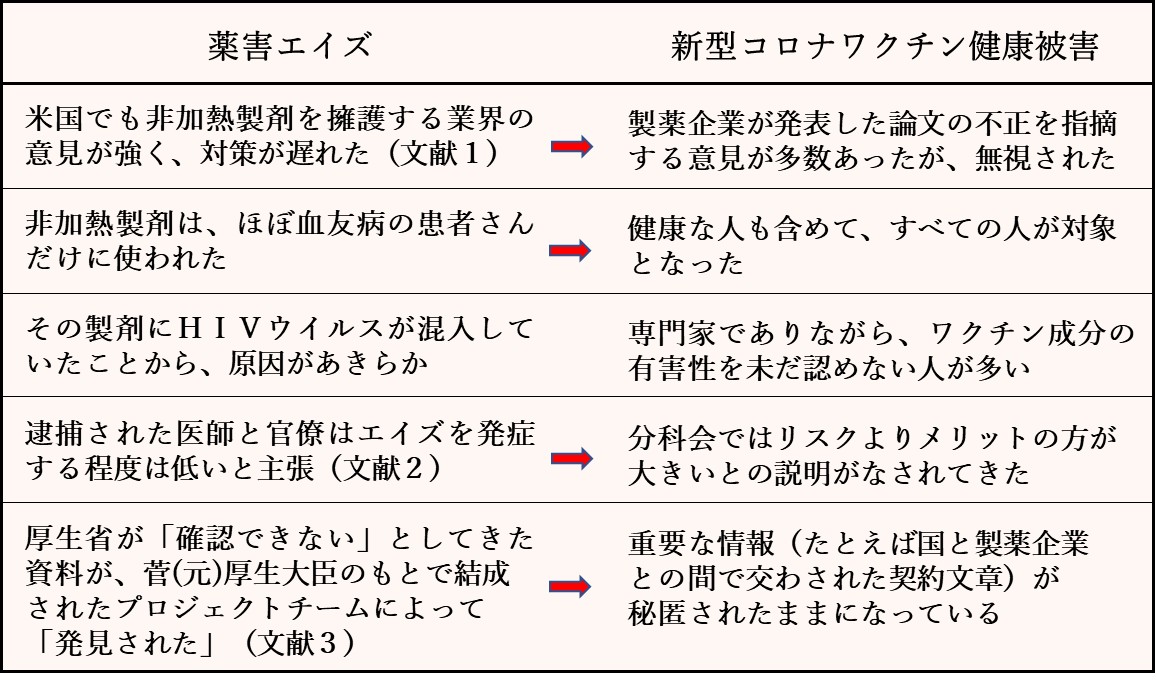
薬害エイズに関して、当時の背景として法律家が指摘していたのは、「製薬会社、行政、大学研究者の三者の癒着」(文献2)であり、「血友病専門医の間の封建的な師弟関係が判断に歪んだ影響を与えた」(文献4)というものでした。
しかし、新型コロナワクチンの場合は、まず背景が大きく異なっています。つまり、「突然、世界を襲った恐怖に、すべての関係者と一般国民が自分のこととして怯えていたこと」、「根強いワクチン神話に人々が洗脳されていたこと」、「mRNAという未知の医療技術に対して、研究者や臨床医たちも正しく理解できないまま、今に至っていること」などではないでしょうか。
このような背景にあって、問題の根本は、新型コロナワクチンを製造販売している企業が世間の信頼を裏切るような形で論文を発表したため、医師、政治家からメディアに至るまで「このワクチンは有効性が高く、副反応は軽い」と思い込まされてしまったことにあります。
次回は総合討論の続きとして、ではどうすれば裁判所の理解がえられ、そして世論を喚起することができるのかを考えます。
【参考文献】
1) Evatt, BL, The tragic history of AIDS in the hemophilia population, 1982-1984. J Thromb Haemost 4: 2295-2301, 2006.
2) 丹羽正夫, 日本における薬害エイズ―厚生省職員の刑事責任をめぐる問題を中心に―, 30(3): 281-306, 1998.
3) 高内寿夫, 行政組織内における責任の構造―薬害エイズを素材にしてー, 白鵬法学, Feb 24, 2017.
4) 水口真寿美, どうしたら薬害エイズを防げていたか〈4つの視点〉, 薬のチェック, 医薬ビジランスセンター, https://www.npojip.org/jip_semina/semina_no1/pdf/094-097.pdf
(2026.2.2)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第19回)?
前回は、厚生労働省とその所管法人「PMDA」の責任についてまとめました。今回は、これら組織に所属する国家公務員に課せられた法律と新型コロナワクチン健康被害との関係を考えます。
かつて私は国立大学に所属していましたので、身分は国家公務員でした。ところが2004年、行政改革の一環で国立大学がすべて法人化されたため、公務員でなくなりました。しかし法律上は公務員とみなされ、すべての規則・法律が引き続き適用されることから、「みなし公務員」と呼ばれるようになりました。PMDAの職員も同様です。
公務員が犯罪を犯すことがあるとすれば、通常は法律に反する行為、たとえば職務に関連してワイロを受け取ったような場合です。これは収賄罪と呼ばれ、公務員でない一般の人には適用されません。
公務員の責任をあきらかにするため、私ごとを少しだけ述べさせていただきます。公務員の役職名には「官」の文字がつき、私の場合は文部教官でした。役職が上がるにつれ職務権限が強くなり、来客も増え、有形無形の誘惑にさらされることになります。教授室にはドアを隔てて前室があり、そこには秘書が控えています。接客の場が密室とならないよう、そのドアをいつも開け放しておくなど、気の抜けない日々だったことを記憶しています。
一方、逆に、公務員が「なすべきことをなさなかった場合(不作為)」も、違法と見做されることがあります。つまり行政機関が権限を行使さえすれば、重大な結果を阻止することができたと認められるような場合です(文献1)。
たとえば水俣病訴訟も不作為が問われた事例です。水俣病とは、化学工場などから排出された有機水銀が海や河川に流れ込み、それに汚染された海産物を長期にわたって食べ続けた住民が、水銀中毒になったという事件です。
これに対して国と県を訴えた裁判では、「海水は海流等により常に移動しており、海域の管理者が海水自体の水質を管理することは事実上不可能」との判断が示されるなど、実際の適応は簡単ではなさそうなのです(文献2)。
現在も各地で訴訟が続いていて、地方裁判所により「国にも責任がある」との判決が出されたり、あるいは「国の責任は認めないが、原告を水俣病と認め原因企業に損害賠償を命ずる」という判決になるなど、判断が分かれたままとなっています。
薬害エイズ事件も注目に値します。血管が損傷すると、体内では出血を止めるための様々な物質が働きだします。その物質の1つが生まれつき欠けている病気に血友病があります。その治療に用いられる血液製剤に、エイズ(HIV)ウイルスが混入していたことから、多くの患者が感染してしまったという事件でした(文献3)。
最終的に医師1名、製薬企業の役員3名、それに旧厚生省の官僚1名が起訴されるに至ったのですが、刑事裁判で「一般の医師がその危険性を的確に認識することは困難であり、エイズを発症し、死亡することを防ぐ措置を検討する職責があった」として、官僚が不作為を理由に有罪となりました。
「世界中の情報をほぼリアルタイムで取得することができていたのは旧厚生省であり、結果的には、官僚主導のリーダーシップ以外、当時の状況を打開するすべはなかった」との判断もあったようです(文献3)。
この考え方は、前々回の中間まとめ第17回で記した「各種分科会における事務局(官僚)と委員{専門家)とのやり取り」にも通ずるところがあります。したがって、新型コロナワクチンによる健康被害についても、厚生労働省とPMDAで中心的役割を果たしていた官僚の責任は、みなし公務員も含め、やはり大きかったのではないでしょうか。
次回は、当ホームページにお寄せいただいたご意見も交え、総合討論とする予定です。反論も含め、さらなるご意見をお待ちしています。
【参考文献】
1) 齊藤彰子, 公務員の職務違反の不作為と刑事事件, 刑法雑誌, Feb 10, 2008.
2) 西埜 章, 行政の不作為責任―規制権限不行使の違法性を中心に―. 明治大学学術成果リポジトリ, Feb 6, 2017.
3) 花井十伍, 薬害の教訓から考える―薬害エイズと血液行政―, 保健医療社会学論集, 27(2), 2017.
(2026.1.26)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第18回)?
前回は、国・厚生労働省のもとに設置された分科会の責任についてまとめました。わかったことは、どの分科会の、どの委員(専門家)も、コロナワクチンの副作用に対して何ら懸念を抱くことなく、事務局の報告をただ聞き流す状況だったということです。
その背景には、委員(専門家)がワクチン神話に強く洗脳されていて、ここ数年で免疫学の概念、とくにワクチンに関する知見が大きく変わったことに思いが至らなった、あるいは意識的に過小評価していたということではないでしょうか。
加えて、前回の記事に対する情報提供があり、分科会の委員の1人が、ファイザー社の元臨床開発統括部長であったことが判明しました。当時、分科会のライブ中継を見ていて、同委員の声高の発言が気になっていたとのことでした。
今回は、各分科会の判断に強い影響を与えた、厚生労働省所管の独立行政法人「医薬品医療機器総合機構」(略称:PMDA)の責任について考察します。
PMDAは、行政改革の一環として2004年に厚生労働省から分離する形で発足した組織で、医薬品や医療機器の有効性や安全性について、治験から承認までを指導・審査する役割を担っています。
職員数は1,056名で、専任のほか厚生労働省からの出向や、大学病院などからの短期出張者で構成され(文献1)、うち70名ほどが医師免許の保有者です(文献2)。私自身、新しい臨床検査法を開発した折に、PMDAに出向き、申請に必要な手続きやデータについてアドバイスを受けた経験がありますが、優秀な技術者集団という印象でした。
一方、厚生労働省のほうは、PMDAが行った科学的な判断をもとに医薬品や医療機器にかかわる権限を行使しています。
2023年3月15日、『新型コロナワクチンを議論する議員有志の会 第1回』という集まりが議員会館でありました。参加者は数名の議員と研究者、それに厚生労働省とPMDAの職員でした。
「正しいエビデンスを得る方法はランダム化比較試験しかない。しかし分科会が引用しているデータは、信頼性に欠ける後ろ向き調査(症例対照試験)に限られている。この点についての見解を!」との、私の問に対する厚労省・PMDA職員の答えは、きわめて官僚的なものでした。実際のやり取りは動画で視聴することができます(文献3)。
「議員有志の会」の中心となっていた某議員は、この会の直前に開催された参議院厚生労働委員会で、ワクチン問題の根幹に迫る質問を行っていました。しかし、それに対する厚労省の説明も、やはり官僚的で、たとえば同議員が「ワクチンの副反応は100種類以上が認定されているが、その作用機序をどのように考えているか?」と質問したことに対し、「承知はしているが、発生機序は必ずしもあきらかでない」などと答えていました(文献4)。
「官僚的」とは、どのようにも解釈できる、そつのない発言という意味です。
ファイザー・ビオンテック社のワクチンが特例承認されたあと、PMDAで何が行われていたのかが論文として残されています(文献5)。そこには、以下のようなことが記されていました。
・国際組織(ICMRA)の共同声明に従い、ワクチンの有効性は免疫原性に基づき
評価すればよい(注釈:中和抗体が上がっていれば、それで十分という考え方)
・副反応の報告がきわめて多くなることを予想し、PMDAではレポートを処理する
100~200人の部隊を編成し、土日も常駐させた
・副反応は、医療関係者を中心に接種していた時期、とくに頻度が髙かった
この論文は、騒動が落ち着いた2023年に発表されたものであり、「あと出しじゃんけん」の感が否めません。その点を加味しても、なお重大な問題点をいくつか指摘することができます。
まず、医療関係者を中心に接種が始まったころの副反応について、「アナフィラキシー反応が、インフルエンザのワクチンに比べ400倍以上も髙かった」というデータが、海外の専門誌に論文として発表されていたことです(文献6)。執筆したのはPMDAの職員でしたが、このことは国民に知らされないまま今日に至っています。私がこの論文の存在を知ったのも、本記事の執筆中でした。
さらに、国際組織の声明に従ってPMDAが定めた『免疫原性に基づく新型コロナワクチンの評価』には、安全性の評価のために少なくとも3000例の被験者に投与すべきことが明記されていたのですが、実施された形跡はありません。
PMDAは官僚組織のひとつですから、上からの指示は絶対であり、板挟みになれば官僚的発言をせざるをえないのでしょう。当時、官僚組織の頂点にいた人は菅 義偉 総理大臣(2020年 9月16日~2021年10月4日)でした。「とにかく早くワクチンを」との焦りから、超法規的な指示を厚生労働省とPMDAに下していたことは想像に難くないところです(文献7)。
コロナワクチンによる健康被害に注意を払わず、ひたすら接種を国民に強いてきた元凶は誰だったのかが、少しずつ見えてきたようです。
次回は、国家公務員(官僚)の法律的責任について考えます。情報をお寄せください。
【参考文献】
1) PMDA, https://www.pmda.go.jp/about-pmda/info-about-pmda/0010.html
2) 病院以外で働く医師(PMDA編)研究開発を影日向になり支える, 記事・インタビュー, 民間医局, May 18, 2020.
3) 【新型コロナワクチンを議論する議員有志の会】 第1回, ニコニコ動画, May 15, 2023, https://www.nicovideo.jp/watch/sm41934237
4) 国会中継, ニコニコ動画, May 10, 2023, https://www.nicovideo.jp/watch/sm42200981
5) 佐藤大作, PMDAでの緊急時の診断・治療手段・ワクチン規制の対応. 医療と社会, 32(1): 37-48, 2023.
6) Iguchi T, et al., Cumulative adverse event reporting of anaphylaxis after mRNA COVID-19 vaccine (Pfizer-BioNTech) injections in Japan: the first-month report. Drug Saf, Aug 4, 2021.
7) 『「いや、百万回だ」ワクチン相を務めた河野太郎氏、菅義偉元首相との「一番の思い出」明かす』, J-CASTニュース, https://www.j-cast.com/2026/01/19511116.html?p=all
(2026.1.19)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第17回)?
前回は、マスコミの責任について考察しました。今回は、国・厚生労働省のもとで行われた各種審議会の責任をまとめます。
当ホームページあてに届いたお便りの中に、「関西地方で2025年6月7日に放映されたテレビ番組で、元分科会長が出演し、当時とまったく異なる発言をしていた」との情報がありました。その番組は現在もyoutubeで視聴することができます(文献1)。
番組中、元分科会長は、「ワクチン副反応の分析はわれわれの分科会ではまったくやっていない。やったのは厚労省にあったワクチン分科会なんです・・・」と発言。司会者が思わず「あらッ!」とひと言。そのあと、「感染防止効果は残念ながらあまりないワクチンです」との言葉もありました。
改めて調べてみると、専門家による委員会が少なくとも次の3つが存在していたことがわかりました。
(A) 新型インフルエンザ等対策有識者会議 基本的対策方針等諮問委員会
→ 内閣官房のもとに2012年設置(文献2)
→ 新型インフルエンザ等対策推進会議 (分科会長: 尾身 茂 氏)と改名し
2021年4月1日に第1回開催
(B) 新型コロナウイルス感染症対策分科会(分科会長: 尾身 茂 氏)
→ 上記委員会(A)のもとに設置され、2020年7月6日に第1回開催(文献3)
同名の会議が2021年4月8日にも第1回として開催(詳細不明)
(C) 厚生科学審議会予防接種・ワクチン分科会
→ 厚生労働省が設置し、2013年4月22日に第1回開催(分科会長: 岡部信彦 氏
(文献4)
→ 同名の分科会が一部改組し、2021年4月8日に第18回開催(分科会長:
脇田 隆字 氏)
以下、当時の出来事と、国のレベルでどのような審議が行われていたのかを時系列で見て行くことにします(会議の名称はA, B, Cで表記、青文字は私の注釈)。
2020年1月15日: 国内で新型コロナ感染症の1例目が確認
2020年7月31日: ファイザー社と政府でワクチン供給に係わる基本合意
2020年7月 6日: 第1回 B分科会 開催
2020年12月31: ファイザー社が行ったランダム化比較試験の論文が刊行
2021年1月 7日: 第9回 A委員会 開催
2021年1月12日: ファイザー社ワクチン接種後に脳出血で死亡した最初の事例
(56歳の男性医師)についての報道(文献5) (注釈:このニュースを報じた
米国の有力新聞社は、これ以降、ワクチンに批判的な記事をいっさい載せ
なくなった)
2021年2月10日: 第18回 C分科会が一部改組し開催
事務局より「ワクチンがメッセンジャーRNAはスパイクタンパク質の設計図と
なる部分でございまして・・・」、「副反応はワクチン群で126例、プラセボ群で
111例と、あまり差はない・・・」、「接種不適格者は、基本的には(従来の)
定期接種と同じになるかと思っております・・・」、「既感染者を接種対象から
除外しないと考えている」などのレクチャーあり。
2021年2月14日: ファイザー社ワクチンに特例承認
2021年2月15日: 第19回 C分科会 開催
事務局より「ワクチンの有効率が新型コロナウイルスに感染歴がない被験者で
調べたところ95%・・・」、「国内治験では死亡とか重篤な有害事象は確認され
なかった・・・ということでございます」、「接種を受ける努力義務が接種対象者
については適用される」、「コロナ感染症とコロナワクチンの副反応は似ているが
・・・、厚生労働省のホームページで対応し、御関心のある方に見ていただく」
などの説明が延々と続いた。
委員からは「予診票の医師の署名欄は、朱肉のハンコということですが、
シャチハタでは駄目だということですか」などの質問あり。
2021年2月17日: 国内1例目のワクチン接種
2021年4月 1日: 第1回 改名A会議 開催
2021年4月 8日: 第1回 改組C分科会 開催
2021年4月12日: 高齢者向けの優先接種開始
2021年5月14日: 第20回 C分科会 開催
事務局より「ファイザー社ワクチン接種後、副反応の重篤報告例が474件、
うち死亡が1件となっております・・・、死亡例については「評価不能」という
内訳に・・・。製造販売業者からの副反応疑い報告は、重篤なものが1,362例、
うち死亡が17という形になってございます」
この説明に対して委員からはとくにコメントなく、ある委員から、
「メッセンジャーRNAワクチンでも血栓症というものはたくさんでてきて
いる・・・」などの発言。(注釈:血栓症が起こるのはアストラゼネカ社
ワクチンであり、mRNAワクチンではほとんど報告例がない)
2021年5月31日: 第22回 C分科会 開催
事務局より16歳以下の接種について、15歳以下と16歳以上の中和抗体価を
記した資料の説明あり。
これに対して委員から、「表中の数値の違いに意味があるのかないのか、
その辺についてご解説をいただければありがたいです」との発言。
2021年9月17日: 第24回 C分科会 開催
委員から「論文からのデータをお示しします・・・、ワクチン接種によって、
抗体に加えてリンパ球も活性化され・・・」、「memory B cellとかmemory
T cellのほうに依存してくる・・・」などの発言。 (注釈:委員側から初めて
の研究データの紹介)
別の委員から「ニューイングランド・ジャーナル・オブ・メディシンに掲載
された論文によれば・・・、3回目接種者は、追加接種者2回接種者と比較して、
感染予防効果が約11.4倍・・・という報告になっております」との説明あり。
(注釈:後ろ向き調査で得られたデータであったが、統計処理の問題に関して
議論が行われることはなかった)
以上、公開されている議事録を、注釈の部分以外は私見を交えず、ありのままに再現しました。国家・厚生労働省のもとに開催された会議で、官僚と専門家が果たした役割が見えてくるのではないでしょうか。なお、とくに裁判で争点になると考えられる発言は赤字にしてあります。
次回は、このワクチンを認可した組織(PMDA)の責任について考えます。
【参考文献】
1) ユーチュブ動画: https://www.youtube.com/watch?v=r5BszP9EKfo
2) 内閣官房, https://www.cas.go.jp/jp/seisaku/ful/taisakusuisin.html
3) 内閣官房, https://www.cas.go.jp/jp/seisaku/ful/yusikisyakaigi.html#3
4) 厚生労働省, https://www.mhlw.go.jp/stf/shingi/shingi-kousei_127713.html
5) Grady D, et al., Doctor's death after covid vaccine is being invastigated. New York Times, Jan 12, 2021.
(2026.1.12)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第16回)?
前回は、「ワクチン接種が、あたかも強制であるかのごとき雰囲気を煽ったのは誰か?」というテーマのもと、「テレビで発する言葉の重さ」について考えました。今回は、その続きでマスコミの責任を取り上げます。
「マスコミ」という言葉は曖昧で対象範囲も広いため、以下、私自身が体験した事実を中心にまとめることにします。
コロナワクチン接種が医療関係者を対象に始まったばかりのころ(2021年初め)、いくつかの週刊誌から取材の申し込みがありました。多くは、ワクチン接種について推進派と反対派の意見を対決させるという主旨の企画でした。私は、当ホームページで紹介していたエビデンスについて解説的な意見をそれぞれ述べました。
ある週刊誌の場合、ワクチン推進派と私のコメントを併記した記事が掲載され、発売と同時にデジタル版にも同じ内容が載りました。すると私のコメントを読んだある作家から、「この記事を撤回しなければ自分の作品の版権をすべて引き上げる」との通知が出版社あてに届いたのだそうです(文献1)。
翌日、デジタル版の記事は、「読者の誤解を招く恐れがあった」との理由で、ネット上から丸ごと削除されてしまいました。
別の有名週刊誌(すでに廃刊)でも、同じようなことがありました。私のコメントを含めてやはり賛否両論が紙面に掲載されたのですが、発売直後に推進派の医師から、私のコメントを「正しくない」と非難するメールが編集部あてに届きました。ネット上にも「ワクチンを否定するような記事を載せたのは許せない」と、出版社を非難する書き込みがいくつかあり、結局、デジタル版に載った記事には、私のコメント部分が消されていました。
ワクチンの副作用として心筋炎が話題になり始めたころのことです。NHKの記者から電話があり、意見を求められました。ひと通り解説めいたことを述べたあと、「心筋炎は無数にある副作用のひとつでしかない。頻度が少ないとの報道で終わりにしないでほしい」と強調したのですが、放送では「心筋炎は極めてまれなので、ワクチンを控える理由にならない」と報じられていました。
全国新聞のいくつかも、私のコメントを記事にしてくれたのですが、しばらくして、それぞれ担当記者から、「社内で配置転換になった」、「会社を辞めることになった」などの知らせが届きました。
知り合いのジャーナリストからは、「某出版社では、社長からワクチン批判の記事は載せるなという指示が出ている」という話も聞きました。
これらのエピソードからわかるのは、ワクチン批判を報じると読者や視聴者からクレームが殺到するという、出版社や新聞社が保身のため自主規制をしなければならない雰囲気が世間に満ち溢れていた、ということです。
かつて民放のテレビ局に務めていたという、あるジャーナリストは、「ワクチン報道に関して政治的圧力があったりするのか」との私の問いに、「民放ではありえない」、「ワイドショーやニュース番組を作っている記者に、科学や医療の専門知識はありません。だからテレビ局の記者に期待するのは無理なんです」と答えておられました。
真実を伝えるというジャーナリズムの使命を、ほとんどのマスコミが果たしてこなかったのは明らかです。しかし見方によっては、マスコミもまた、同調圧力に屈した被害者だったのかもしれません。
次回は、政府・厚生労働省の責任について考察します。
【蛇 足】
このような雰囲気にあっても、私の知る限り『週刊大衆』、『週刊ポス』、『女性セブン』の各誌は、正しい記事を載せ続けていました。
【参考文献】
1) コロナワクチンを「絶対にうちたくない」と医師が言うワケ 感染予防効果なし, 阿修羅, Jul 14, 2024, http://www.asyura2.com/23/iryo12/msg/779.html
(2026.1.5)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第15回)?
今回も、「中間まとめ」の総まとめとして、ワクチン接種があたかも強制であるかのごとき雰囲気を煽ったのは誰だったのか、について考察を続けることにします。
当ホームページあてに、この問題についての貴重なご意見が何通か届きました。要約すれば、「教師に対する圧力は、やはり教育委員会だったのではないか」、「マスコミで穏やかな強制が発信されていた」、「日本政府が諸外国から接種率を上げるように迫られたのではないか」・・・などのご意見でした。熟読の上、考察に反映させていただきます。
さて前回、ほとんどの医師がコロナワクチンは打った方がいい、と考えている旨を記しました。医師は、大学の教育で「○○感染症が撲滅できたのはワクチンの恩恵」などの講義を繰り返し受けるのですが、そのことも理由のひとつかもしれません。「ワクチン」が、絶対的な医療を表す言葉であるがごとき刷り込みが行われる、というわけです。
・・・・実は、「ワクチンで撲滅」の逸話も、当ホームページの2025年2月10日と2025年3月31日付け記事で記したとおり、いまだ明確なエビデンスは存在していないのですが、現役の医師には伝わっていません。
2020年(パンデミックが始まった年)の暮れ、早々と『mRNA型ワクチンの優秀さ?』を示す論文がファイザー社から投稿され、著名な学術専門誌に掲載されました。その専門誌ニュー・イングランド・ジャーナル・オブ・メディシンは、臨床医学の最高峰とされ、そこに掲載された論文には「水戸黄門の印籠」のごとく、世界中の医師がひれ伏す、というほどの存在です。
ところが、同誌の編集長を務めていたマーシャ・エンジェル医師は、退任後に本を執筆(文献1)。世界的な製薬企業による論文には数々の不正があり、それらを掲載し続けてきた同誌にも重大な責任があるとの告発を行っていたのでした(文献1)。当時、タイムズ紙が同氏を「もっとも影響力がある25人」の1人に選ぶなどメディアの評価は高かったのですが、なぜか医師たちがこの本に目をとめることはありませんでした。
いずれにしても、多忙な第一線の医師たちには、日々、英文で発表される膨大な数の論文に目を通す時間がありません。通常は、各分野で専門家と目される医学研究者が論文を読み、要約した内容が講演会などを通して第一線の医師たちに伝えられます。
ところがコロナワクチンの場合、事態があまりに急速に進行したことから、第一線の医師たちに情報が伝わったのは一般の人たちと同様、テレビなどで有名医師が語る話によるものだったようです。
では、責任は専門家と称するこれら有名医師たちにあったのでしょうか?
いまから15年ほど前、イタリアの都市ラクイラで群発地震が続いていたため、6人の専門家が招集され分析が行われました。その結果が「大きな地震にはつながらず、避難する必要はない」との言葉で地元テレビで報じられました。ところが6日後、悲劇的な大地震が同地方を襲い、逃げ遅れた多数の市民が死亡したことから、6人の専門家は過失致死罪で告発され、禁固刑が言い渡される事態となりました。
最終的に、イタリアの最高裁で「予測は不可能だった」として無罪になったですが、科学者の責任がどこまで問われるのかを考えさせられる出来事だったのです(文献2)。
科学の進歩は、常に「多くの間違った見解」と「わずかな正解」が入り交じり、歴史の中で真実が見えてくるという繰り返しでした。したがって科学者が間違った見解を発表するたびに罪を問われては、科学は進歩しないことになってしまいます。
イタリアの出来事で、もうひとつ追記しなければならないことがあります。6人の専門家のほかに地方政府の高官が告発され、「専門家の見解を歪曲し、テレビで安全宣言した」として最高裁の判決で、だだ一人有罪を言い渡されていたことです。6人の科学者が直接、テレビで見解を述べていたわけではなかったのです。
このエピソードでわかるのは、立場や身分がどうあれテレビで発する言葉の重さです。週刊誌報道によれば、「新型コロナワクチン関連専門家・テレビ番組出演本数ランキング」なる調査データがあり、上位10人のうち4人が、以前、ワクチンメーカーから講演料、寄付金などを受け取っていたとのことです(文献3)。このようなデータは、情報公開制度によって得られるものであり、正式な手続きを経て取得したお金が対象となっているはずです。その場合、違法性はないのですが、当然、そこには忖度が働きます。
当時、テレビで繰り返し報じられた政府専門家会議の委員の発言も気になります。
各種委員会での個々の委員の発言は、ごく一部しか議事録として公表されておらず、「副反応が非常に高い頻度で起こるということが言われている・・・」などの記述がわずかに認められるにすぎません(文献4~6)。
ワクチン接種の是非に関して、どの会議でどのような議論がなされたのか? テレビで語られた委員の話は政府の公式見解だったのか? もしそうでなければ誰の責任で発言がなされたのか? などについて情報公開を求めていく必要があります。
テーマが複雑なため、考察はもうしばらく続けることになりそうです。さらなるご意見をお待ちしています。
【備 考】
頻回にテレビで報じられた「新型コロナウイルス感染症対策分科会」なる組織は、政府の新型インフルエンザ等対策閣僚会議の下に設けられた「新型インフルエンザ等対策有識者会議」が開催する分科会のひとつ。その第1回会合が2020年7月6日に開催されたが、同時に「基本的対処方針等諮問委員会」が同年3月27日から開かれていて、しかも委員のうち7人が両方に属しているなど責任の所在が判然としない。
【参考文献】
1) マーシャ・エンジェル著, 栗原千絵子・斉尾武郎=共監訳, 『ビッグ・ファーマ、製薬企業の真実』, 篠原出版新社、2005.
2) Cartlidge E, Italy's supreme court clears L'Aquila earthquake scientists for good. Science, Nov 20, 2015.
3) ワクチン会社から謝礼を受け取っていた番組コメンテーター医師の実名, 週刊ポスト, 5月7・14日号, 2021.
4) 新型インフルエンザ等対策有識者会議 基本的対処方針等諮問委員会(第9回)議事録, Jan 7, 2021, https://www.cas.go.jp/jp/seisaku/ful/shimon9_2.pdf
5) 新型コロナウイルス感染症に係るワクチンの接種について, 内閣官房, 厚生労働省, Feb 9, 2021, https://www.cas.go.jp/jp/seisaku/ful/bunkakai/wakuchin_sesyu.pdf
6) 現在の新型コロナワクチンの感染予防効果のエビデンス, アドバイザリーボード提出資料, May 6, 2021. https://www.mhlw.go.jp/content/10900000/000796742.pdf
(2025.12.29)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第14回)?
今回は、「中間まとめ」の総括として、コロナワクチンによる健康被害の元凶がどこにあったのかを考察します。責任の所在を明確にすることは、国家賠償を求めて裁判を起こす際に必須です。
コロナ禍以前にも、ワクチンによる健康被害を巡る裁判は少なくありませんでした。たとえば、平成3年に最高裁で次のような判決が下されています(文献1)。裁判の判決文ですから非常に複雑で難解のため、意味を逸脱しないよう注意しながら簡潔にまとめてみます。
「1週間前から間質性肺炎に罹っていた子供がインフルエンザワクチンの接種を受け、翌日に死亡した」という遺族の訴えです。論点は接種を受けてはいけない人(禁忌者)を接種会場で判断できなかったのかということで、次のような指摘がなされました。
『問診票などを用いることは許容されるが、最終的に担当医師は被接種者にわかりやすい言葉を使って確認するなどして、総合的に判断する義務がある。したがって、適切な問診を尽くさなかったために禁忌者の識別を誤った場合、医師に過誤があったものと推測するのが妥当。担当医師の使用者である地方公共団体にも責任があり、現代の医学水準から予知ができなかったことを立証しない限り、不法行為責任は免れない・・・』
しかし、医師にとってワクチン接種は、いわば国家の事業への協力であり、結果について責任を問われることはないとの認識が一般的で、医師向けの専門誌にもそのような記述がなされています(文献2)。
注目すべきは、新型コロナワクチンの問診票(文献3)が、インフルエンザワクチンの問診票(文献4)と、ほとんど同じ内容であったことです(参考文献の青文字の部分をクリックすると問診票(予診票)の実物を見ることができます)。そのため、ほかに何を問診で確認すればよいのかが明確でありませんでした。
接種に協力した医師の多くは、従来のワクチン接種と同じ気持ちで、とくに身構えることなく対応していたものと推測されます。唯一、従来のワクチン接種と異なっていたのは注射の部位でしたが、事前に動画や資料による説明を受けていたはずで、そのことでとくに問題は生じなかったと思われます。
つまり一般論としては、コロナワクチンの接種を担当した医師の責任を問うことは困難と考えられるのです。
当時(そして今も)、ほとんどの医師は「コロナワクチンは必須」と考えており、患者さんから問われれば「受けるべき」と答えてきたようです。実際、当ホームページ宛てに届いたお便りの中にも、「アレルギーがあって接種を受けたくなく、その旨を職場に伝えるための診断書を医師に求めたが断られた」などの訴えもありました。
過去5年間、勤務先や学校内における「ワクチンを受けなさい」との同調圧力は尋常でなく、そのために退職を余儀なくされたという訴えも少なくありませんでした。子育て中のお母さんからは、「自分は受けたくなかったのに姑、姑からのきつい言葉が辛かった」とのお便りもありました。
一方、接種を拒否して解雇されそうになった教員が、地元の教育委員会に問い合わせたところ、「強制はいっさいしていないので安心してください」と、逆に励まされたというお便りもありました。
厚生労働省のホームページには、「原則としては接種勧奨の実施と接種を受ける努力義務を適用する」と、いささか高圧的な文章が掲載されていましたが、一方、市民からの問い合わせに対しては「接種は強制ではなく、最終的にはあくまでご本人が納得した上でご判断いただく」との回答がなされていました(文献5)。
このように国の説明は曖昧だったわけですが、ではワクチン接種があたかも強制であるかのごとき雰囲気を煽ったのは誰だったのでしょうか? 次回は、この問題について体験談も交えて考察します。(ぜひ、ご意見をお寄せください)
【参考文献】
1) 川島陽介, No.110/ワクチン接種(予防接種)における医師などの注意義務ー最高裁H3.4.19判決、最高裁S51.9.30判決ー, 弁護士法人ふくざき法律事務所, Dec 1, 2022.
2) 川崎翔, 【識者の眼】予防接種の副反応による健康被害の責任の所在は?, 週刊日本医事新報, 5078号, Sep 19, 2025.
3) 新型コロナワクチン接種の予診票
4) インフルエンザワクチン予防接種予診票
5) 吉田加奈子, 新型コロナワクチン接種と人権, TOKYO人権, 第93号、Feb 28, 2022.
(2025.12.22)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第13回)?
ここまで12回にわたり、コロナワクチンの問題点について「確かなエビデンス」のまとめを行ってきました。目的は、裁判を有利に進めるための準備です。今回
まず事件の背景について理解を深めるため、少し時間を遡って事の発端がどのようなものであったのかをみておきましょう。
いまから30年ほど前、mRNAをワクチンに応用できるのではないかと考えて実験を行っていた研究者が世界中に何人かいました(文献1)。その一人、米国カリフォルニア州の研究所に大学院生として在籍していたR.マローン氏は、mRNAを脂質微粒子膜に包んで、カエルに注射するという実験を世界で最初に行っています。しかし同氏は、「このワクチンで作られるたんぱく質は危険だから」という謎の言葉を残して研究から手を引きました。
このアイデアを諦めることなく実用化するための研究を続けたのが、2008年にドイツでビオンテック(BioNTech)社を創設した2人の科学者U.サヒンとO.トゥレジの夫妻です。同じころ、がんの治療にmRNAを応用する研究を行っていたポーランドの2人の科学者が、実験に成功していました。
ビオンテック社は、この2人と共同研究を開始するとともに、のちにノーベル賞を授与されるK.カリコ氏を社員として採用します。2020年、新型コロナに対するワクチンの開発に成功すると、臨床試験と製造販売を行なうためのパートナーとして、米国ファイザー社に協力を呼びかけました。それに応じた同社の社員は、ドイツからサンプルを持ち帰り、直ちに動物実験を開始したのでした。
同じ時代、2010年に創設された米国の企業モデルナでも、mRNAを利用したジカウイルス感染症のワクチンを完成させていました(文献2)。
2020年の正月、同社の経営者S.バンセル氏は家族と休暇中で、海外のホテルでいつものようにウォール・ストリート・ジャーナル紙を読んでいました。目にとまったのは、「中国の武漢で謎の肺炎、原因不明」という記事でした。ビジネスマンとして百戦錬磨の彼は、早速、中国武漢発の国際線をグーグルで検索し、世界中のほぼすべての都市に飛んでいる事実を知り、驚愕します。
1月7日、共同研究を行っている国立衛生研究所(NIH)のワクチン担当B.グラハム氏にメールを打ちました。3日後、NIHもmRNA型のワクチンが有力であることに賛同する旨の返信を受け取ると、その日のうちに、同社でmRNAワクチンを担当する敏腕技術者に転送。直ちに開発に取りかかることになりました。
・・・と言っても、ウイルスの遺伝子配列を入力するだけで、ワクチンが出来上がる体制がすでに出来上がっていたのです。1月13日、新型コロナウイルスの遺伝子配列が中国の研究者によって解読され、科学専門誌ネイチャーに論文が掲載されました。
25日後の2月7日、最初のワクチンが早々と完成しました。検証を終えた3月2日、バンセル氏は、トランプ大統領と面会する機会をえます。大統領もコロナ対策で焦っていて、大手製薬企業の経営者たちとの会合を予定していたところだったのです。同氏は、創業したばかりの小さな会社の経営者でしたが、すでにワクチンは完成し、人間での試験も予定していることを説明したところ、大統領より直々に支援が告げられたのでした。
3月16日、このワクチンは、人類で初めて人体に注射されました。注射を受けたのは、フェイスブックでボランティア募集記事を見て、自ら応募した女性でした。
4月29日、米国政府は、新型コロナワクチンや治療薬の開発を加速するため、モデルナ社など6社に莫大な資金を提供する計画(ワープスピード作戦)を発表します。この作戦を実行するための組織は、制服を着た数十人の軍人も加わり、まるで軍隊の作戦チームのようでした(文献3)。
臨床試験も佳境を迎えた同年の初秋、新たな問題が持ち上がりました。ファイザー社もモデルナ社も、マイノリティ(黒人やヒスパニック系)の試験参加者が少なすぎると、当局からクレームがつけられたのです。
資金力で勝るファイザー社にとっては簡単なことでした。しかし一方、同社は臨床試験の途中で、逐次、感染者をカウントするなど「ランダム化比較試験」の原則から外れる方法をとっていたため、規制当局から指摘を受け、手順の変更を迫られていました。
その変更を、ようやく当局から許可されたのが11月3日。これは同社の臨床試験が最終段階に入ったことを意味します。奇しくもこの日は、トランプ大統領が再選を目指した選挙の投票日でした。トランプ氏は「意図的に許可を先延ばしにしたのではないか」と、激怒したと伝えられています(結果的に選挙で大敗)。
11月8日の日曜日、ファイザー社では重役たちが集まり、臨床試験の結果について統計担当者から報告を受けることになっていました。報告は「感染者がプラセボ群で90人、ワクチン群は4人だけだった」との内容で、部屋中が歓喜に包まれました。重役の一人は、「19人、それとも90人?(nineteen or ninety ?)」と聞き返したほどでした。
この話が本当であれば、製薬企業の重役陣は、臨床試験が最終段階を迎えるまで結果を知らなかったことになります。そして、なぜか感染者の人数が、のちに論文で公表される値と大きく異なっていました。
その後、モデルナ社から発表された情報も「感染者がプラセボ群で90人、ワクチン群で5人」と、奇妙な一致を示していました。・・・FDAは、早くも12月1日と18日に、それぞれファイザー・ビオンテックとモデルナ社のコロナワクチンの緊急使用を許可。
以上がメディアで報じられた背景のすべてです。新型コロナのパンデミックに翻弄される政治家の狼狽ぶりと、製薬企業の思惑、さらに規制当局の関与をうかがい知ることのできるエピソードですが、その後、製薬企業によって発表される論文に投げかけられる数々の疑問と、どのように結びつくのかは想像するしかありません。(次回に続く)
【参考文献】
1) Aygün I, et al., The forerunners and successful partnerships behind the BioNTech mRNA vaccine. J Appl Genet, Oct 20, 2023.
2) Elton C, The untold story of Moderna's race for a COVID-19 vaccine. Boston, Jun 4, 2020.
3) LaFraniere S, et al., Politics, science and the remarkable race for a coronavirus vaccine. New York Times, Nov 30, 2020.
(2025.12.15)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第12回)?
ここまで11回にわたり、新型コロナワクチンによる健康被害について、裁判を想定した科学的根拠のまとめを行ってきました。今回は、国内ですでに始まっている裁判の現状を考えます。
以下の2つの表は、国家賠償を求めた裁判の事例をまとめたものです。ネット上で確認がとれたもののみ掲載しており、すべてを網羅したものではありません。また、国と製薬企業との間で交わされた新型コロナワクチン契約書の公開を求めた裁判や、レプリコン型ワクチンを巡る係争、あるいは接種直後のアナフィラキシーに対する処置を巡る訴えなどもありますが、これらも除外しました。
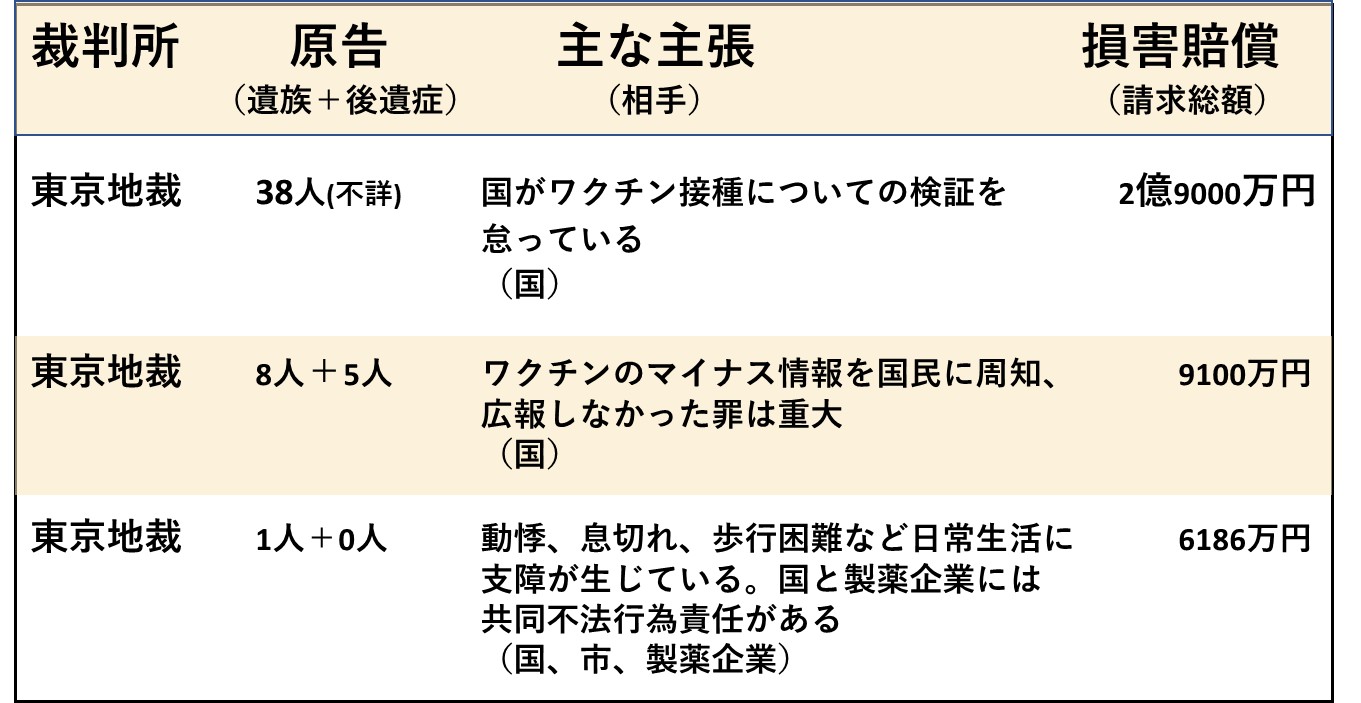
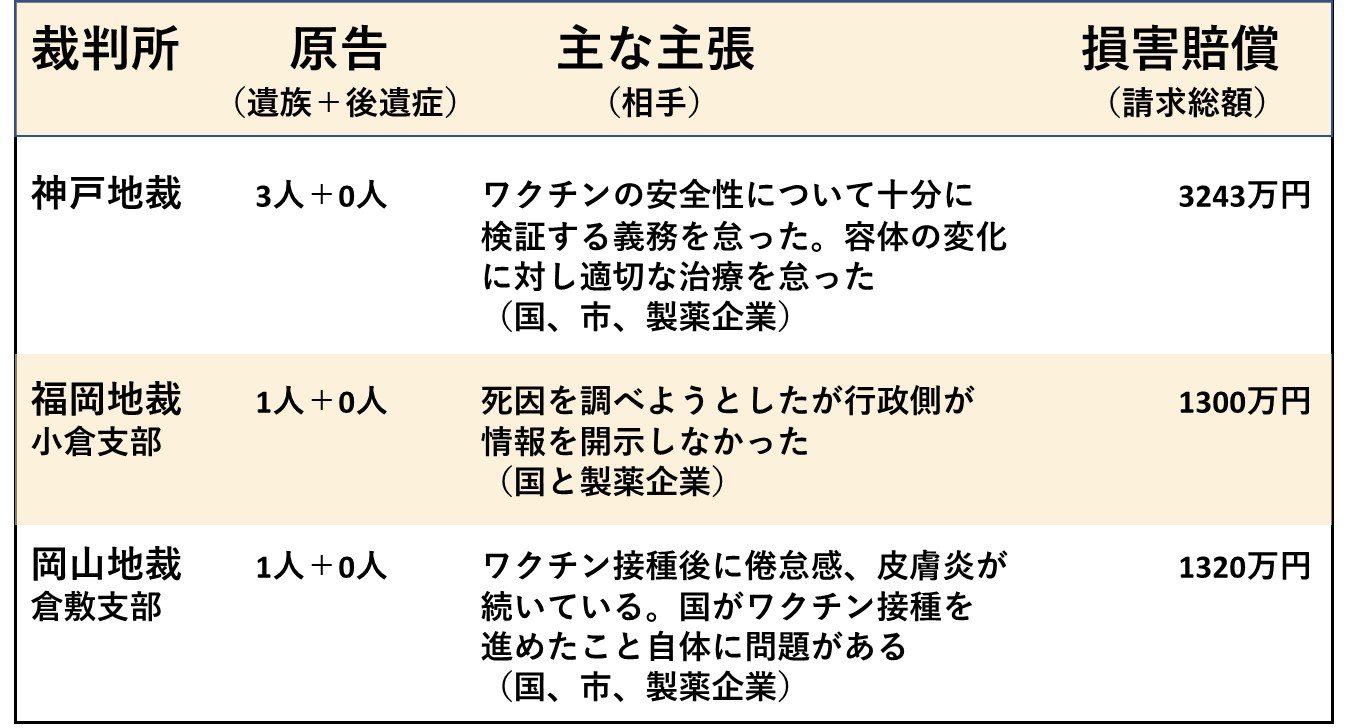
いま裁判を起こしている人たちが訴えているのは、単に健康被害の補償を求めているだけでなく、十分な臨床評価を経ずに緊急承認されたワクチンだったにもかかわらず、リスクの説明や情報開示がなされないまま、世論を煽るような形で接種が勧められてきたことに対する「怒り」です。
ただし、国や製薬企業は、さまざまなデータを駆使して、あるいは独自の論法を用いてその正当性を強力に主張しています。たとえワクチンの欠陥や行政の不当性を訴えたとしても、医学や統計学の専門家を総動員して反論してくることが予想され、裁判を有利に進めるのは簡単でありません。
少なくとも、コロナワクチンによって健康被害を受けたことを主張するには、自身の症状が副作用としてすでに認知されているものであることや(中間まとめ第1回を参照)、接種後から発症(または死亡)に至るまでの経過に矛盾がないこと(同じく第4回参照)、その間に医療機関で受けた検査のすべてのデータが保存されていて、医師の診断書も得られること、などが必須要件です。
また、ほとんどの人が、(症状の有無にかかわらず)新型コロナに感染してしまっていることから、感染の後遺症ではなく、真にワクチンの副作用であることを自ら示す必要があります。具体的には、抗体検査を受けていて、その結果がN抗体が陰性、かつS抗体が陽性でなければなりません。両方とも陽性だった場合は、新型コロナウイルスに感染していたことになり、「ワクチンが原因」と主張するのは困難だからです。
これらの情報が、救いのないまま諦めてしまっている多くの被害者の方々に届くことを願うばかりです。次回は、「中間まとめ」の最終回として、誤ったコロナワクチン行政の責任がどこにあったのかを考察します。
【参考文献】
なし
(2025.12.8)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第11回)?
新型コロナワクチンの接種によって、深刻な健康被害を受けた人が少なくありません。当ホームページあてにも、そのような方々からのお便りが数多く届いていますが、多くは医師や行政に救いを求めても理解が得られなかったなど、悲痛な心境を綴ったものでした。
それにもかかわらず、ワクチン被害の問題について世論が盛り上がることはなく、すでに風化してしまった感さえあります。当ホームページあてに届いたお便りの中に、コロナ対策の中心となっていたある専門家が最近、テレビ番組に出演し、「感染を防ぐ効果は残念ながらあまりないワクチンです」、「重症化予防効果はある」などど語っていた、と耳を疑う情報提供もありました。
今後は、裁判を通じ実態が明らかにされ、世論を喚起するきっかけになることに期待するしかなさそうです。
当ホームページでは、これまで「新型コロナワクチンの問題点」に関する確かな情報を掲載してきましたが、情報が多岐にわたり、あまりに膨大になってきたことから、本質が見えなくなってしまっているのではないか、との懸念もあります。そこで特に重要で、かつ科学的な方法で実証がなされているデータを厳選し、ここまで10回にわたりまとめを行ってきたところです。
もちろん、これらのデータ以外にも、ワクチン被害を懸念する人々が関心を寄せている情報は多数あります。いずれも興味深く、信ぴょう性も高いのですが、確固たる実証データがまだないため、容易に反論を許してしまいそうなものも少なくありません。
たとえば「超過死亡」です。過去の死亡統計をもとに、新型コロナの流行期に総死亡率が異常に高まっていたことを示す言葉です。しかし、総死亡率は年々変化しているものであり、推計が難しく、計算方法も確立しているわけではありません。そのため論文によって結論が異なっていて、反論の余地が多々あるのです。
総死亡数のうち、ワクチンが原因となった死亡数を特定するのは、さらに困難です(詳細は当ホームページの2021年12月27日付、および2023年3月20日付の記事を参照)。
「がんが増えた」との研究報告も多数あります。私自身が行った計算でも、「何種類かのがんがあきらかに増えている」という結果でした。しかし、がんの原因は無数にあり、ワクチン以外の影響を取り除くのはほとんど不可能です。また、がんの潜伏期は一般にもっと長いと考えられていることから、反論の余地はいくらでもありそうです(詳細は当ホームページの2024年6月10日付、2024年12月23日付、および2025年1月6日付の各記事を参照)。
「抗体依存性感染増強(ADE)」も、特殊な専門用語でありながら、ワクチン問題に関心を寄せる人々の関心事となっています。ワクチン接種によって期待されるのは、ウイルスを無毒化する中和抗体が体内にできることですが、それ以外にも免疫反応の過程でさまざまな抗体ができてしまいます。多くは無害で無用なものですが、一部がウイルスの増殖を促進し、感染を増強してしまうという現象です。
昔から知られていた現象なのですが、実際に起こったことを細胞レベルや分子レベルで証明するのは難しく、コロナワクチンとの関係を示すデータも、いまのところ発表されていません(詳細は当ホームページの最下段にある目次のQ7(3)から参照可)。
長期的な影響で懸念されるのは、「妊娠中のワクチン接種」で胎児や生まれてくる子供に悪影響はないのかということです。母乳中にワクチンの成分が検出されたというデータは存在しますが、それが赤ちゃんにどのような影響を与えたかは不明です。
奇形の原因になるのではないかとの懸念も多くの人が抱いているものですが、「いまのところ影響は出ていない」と結論した論文はあるものの、真相の解明には長い年月がかかりそうです(詳細は当ホームページの2021年11月29日付の記事【妊娠・出産・育児を考える】を参照)。
これらは、いずれも現時点で科学的根拠が不十分なため、係争のテーマとするのは避けたほうがよさそうです。
すでにワクチンによる健康被害に対して、国家賠償を求める裁判もいくつか始まっています。確かな情報に基づいて、正しい結論が導かれることを願うばかりです。
【参考文献】
なし
(2025.12.1)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第10回)?
前回は、「製薬企業が行ったランダム化比較試験は、すでにデータ収集の段階で重大な疑義があった」ことをまとめました。今回は「同社が発表した半年後の評価に関する論文」についてです。
ファイザー社は、「6カ月後のコロナワクチンの評価」と題する論文を発表しています(文献1)。同社が行ったランダム化比較試験については、2020年12月31日付けで論文が発表されていましたから(文献2)、その第2報ということになります。
第2報の目的は、ランダム化比較試験が行われた期間と、そのあと目隠しを外した期間の合わせて6ヵ月間のデータを評価すること、と記されています。「目隠しを外す」というのは、ワクチン群とプラセボ群に割り当てられた人々に対し、それぞれどちらの群であったかを明かしたという意味です。またプラセボ群の人たちには、希望に応じてワクチン接種が行われました。
結論は、「6ヵ月でワクチンの効果は若干減ずるものの、感染を予防する効果は依然として91.3%と高い」というものでした。しかし、その背景には多くの疑問が潜んでいます。
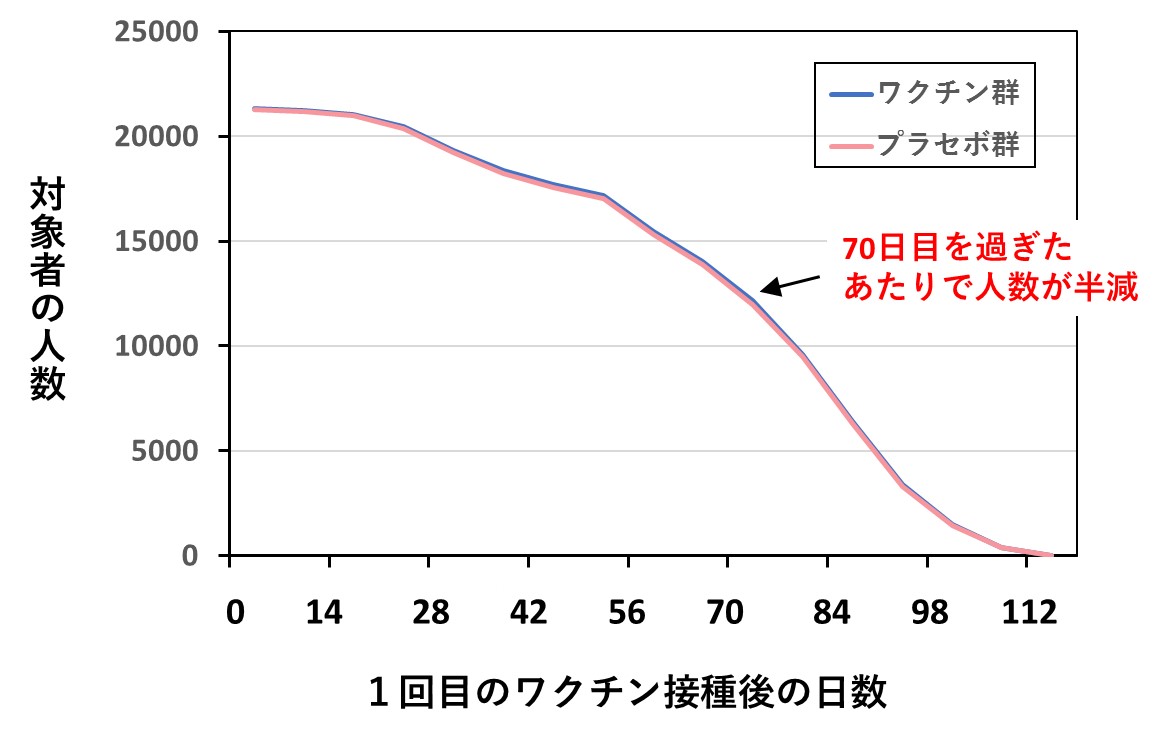
たとえば、ランダム化比較試験の対象人数と調査期間が、なぜか第1報の説明とかなり異なっています。このことによって対象者の範囲が変わってしまい、結果に影響を与えていないのか、気になります。
また、調査期間中の総死亡数が、ワクチン群で15人、プラセボ群は14人だったと記述されています。「総死亡数」は原因を問わず調査期間中に亡くなった人の総数で、コロナ感染で亡くなった人も含まれていますが、いずれも同試験の評価委員によってワクチンが原因ではないと判定されたとのことです(文献3)。
このワクチンが、真に人々の健康増進に寄与するものであれば、当然、総死亡数はワクチン群のほうで少なくなっているはずです。しかし僅差とはいえ、むしろ多くなっているのは、「健康で長生きをするため」という医療への期待に背く結果です。加えて、目隠しを外したあと、プラセボ群でワクチンを接種した人のうち2名が死亡したとも記されているのです。
同論文の最後には、「ランダム化比較試験を終了し、目隠しを外した期間中にプラセボ群の人々にも必要に応じてワクチンを接種した。その上で、ワクチンを接種したすべての協力者を今後2年間、追跡する予定である」とありました。
このような倫理的判断に対して誰も反対することはできませんが、せっかくランダム化比較試験として調査がスタートしたにもかかわらず、「比べる相手」を途中で意図的に抹消してしまった、とも言えるのではないでしょうか。
たとえば2年後、ワクチンを接種した人たちにがんが増え、副作用ではないかと指摘されても、「がんは年齢とともに増えるもの」との説明で、批判を封じることができてしまいます。
第2報の発表によって、疑惑がさらに深まっています。次回は、ワクチンによって健康被害を受けた人たちによる裁判を想定して、「反論を許さない確固たるエビデンス」の総括を行う予定です。
【参考文献】
1) Thomas SJ, et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med, Sep 15, 2021.
2) Polack FP, et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med, Dec 31, 2020.
3) 文献1のSupplementary Appendix, Table S4 Causes of death from dose 1 to unblinding (safety population, ≥16 years old).
(2025.11.24)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第9回)?
前回は、「製薬企業が発表したランダム化比較試験の論文には、データ提示の仕方にさまざまな疑念がある」ことをまとめました。今回は、「そのデータを収集したプロセスにも疑義が指摘されている」という問題についてです。
疑義の指摘は、英国の医学専門誌の副編集長を務める人が発表した記事でなされたものでした(文献1)。ファイザー社の論文には、ワクチン群とプラセボ群のそれぞれについて協力者数と感染者数が記されていましたが、実は感染の疑いがあったにもかかわらず3,410人にPCR検査がなされず、除外されていたとの内容でした。
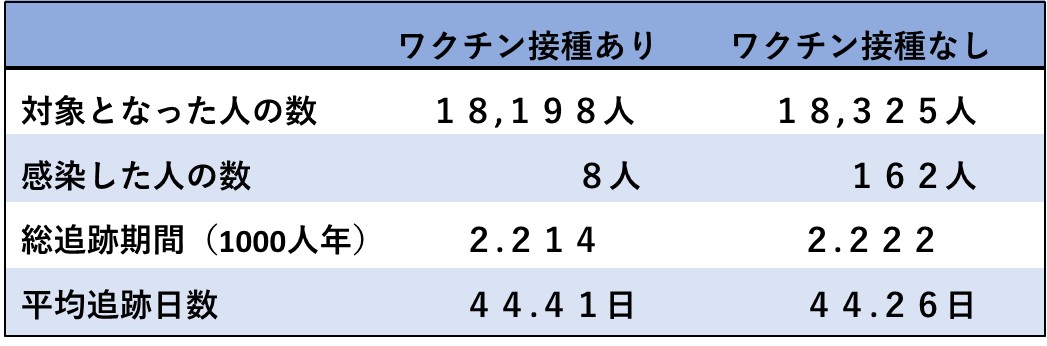
その記事の元になっていたのは、同社が米国食品医薬品局(FDA)に提出した書類です(文献2)。そこには、合計3,410人(ワクチン群1,594人、プラセボ群1,816人)がPCRで未確認だったため分析から除外したと、確かに記されています。
論文に掲載されるべき協力者の一部が、あいまいな理由で除外されていたのであれば、肝心の有効率の計算結果もまったく違ったものになるはずです。前々回(11月10日付)のまとめ記事で掲載した数値を、この情報に合わせて訂正すると、以下のようになります。
ワクチン群 プラセボ群
協力者数 18,198人 18,325人
感染者数 8+1598人 162+1816人
論文中に記されていた有効率は95.0%でしたが、この数値を使って計算をしなおすと、わずか18.8%になってしまいます。
このことについて同社は、「未確認の意味は、症状に疑いのある3410人にPCR検査がなされたが陽性でなかったということ」と説明しています。原文中、「未確認」はunconfirmedとなっているのですが、この言葉は医学では「発熱などの症状があったが検査は行われなかった」という意味で使われるのが普通です(文献3)。
同じ英国の医学専門誌に、もうひとつ衝撃的な記事が載っています(文献4)。
ファイザー社の第III相試験(発売前の最終臨床試験)が始まったのは、新型コロナウイルスが蔓延し始めた2020年の夏。全世界153の組織が協力し、44,000人のボランティアを集めるという一大事業でした。
協力したのは医療機関だけでなく、最近、国内外で増えている「治験請負会社」でした。そんな会社のひとつに治験コーディネーターとして勤務するブルック・ジャクソンさんは、15年以上の経験があるベテランで、この会社に引き抜かれたばかりでした。
彼女は、この会社で仕事を始めてすぐ、治験のやり方に重大な疑義があることに気づきます。たとえば副作用があれば、外部の治験調整機関に直ちに報告することになっていましたが、放置されたままでした。
もっとも深刻だったのは、本物のワクチンが収められたほうのケースに協力者のID番号が刻印されていたことでした。このままでは、誰が本物のワクチン接種を受けるのかわかりますから、恣意的にプラセボ群に割り当てられた人と入れ替えることもできてしまいます。このような操作は、実は、これまで多くの医薬品の臨床試験で繰り返されてきた不正の手口でした。
9月24日、彼女は、これらの問題点を上司に伝えましたが、受け流されてしまったため、翌25日、当局(FDA)に宛てて告発状を送りました。そして、その日の午後、彼女は会社から解雇を告げられたのです。
この話は、すべて医学専門誌に記事として掲載されていたものです(同文献4から彼女のインタビュー動画を視聴することができます)。同記事は、そのころ会社を退職した2人の元同僚にも取材を行い、「彼女の言っていることはすべて正しい」、「477人が新型コロナの症状を訴えていたにもかかわらず、PCR検査が行われなかった」との新証言を得たことも記されていました。
このような出来事は、果たして治験が行われた一ヵ所だけのものだったのか気になるところです。次回は、製薬企業が発表した「ワクチン接種後6ヵ月間の効果と安全性」と題する論文の疑義についてまとめます。
【参考文献】
1) Doshi p, Pfizer and Moderna's "95% effective" vaccines - we need more details and the raw data. BMJ Plogs, Jan 4, 2021.
2) Pfeizer and BioNTech, Vaccines and related biological products advisory committee meeting. FDA Briefing Document, Dec 10, 2020.
3) Zhan C, et al., Estimating unconfirmed COVID-19 infection cases and multiple waves of pandemic progression with consideration of testing capacity and non-pharmaceutical interventions: a dynamic spreading model. Inf Sci, Jun 6, 2022.
4) Thacker PD, Covid-19: researcher blows the whistle on data integrity issues in Pfizer's vaccine trial. BMJ, Nov 2, 2021.
(2025.11.17)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第8回)?
前回は、「製薬企業が発表したワクチンの有効率には、意図的な情報操作が行われていた」ことを示す論拠をまとめました。今回は、その続きです。
ファイザー社が発表した論文には、ランダム化比較試験の結果がグラフとしても提示されています(文献1)。次の図は、そのグラフを参考に、私が作成したイラストです。
一目見た印象からは、「製品の優秀さ」が見事に表現されています。しかし論文中、「縦軸は調査期間中に新たに感染した人の割合を、順に足し算していった値」と記されているなど、あえて難解な表現がなされています。しかも文献1と2に記載されたどの数字とも計算結果が一致せず、このグラフが果たして正しいのか、疑問も残ります。
グラフの形をよく見ると、最後のほう(右端)が横這いになっていて、両群の差がなくなっている(ワクチンの効果がなくなっている)こともわかるのですが、グラフ全体の形に目を奪われ、つい見逃してしまいそうです。
次の図は、ファイザー社が米国食品医薬品局(FDA)に提出した書類に掲載されていた数値を前述のグラフに追記したものです(文献2)。
赤字で示したこの数値を、グラフにしたものが次の図です。
このグラフの解釈もいささか難解です。大規模な臨床試験では、実薬群とプラセボ群の協力者全員に対して、いっせいに試験(ワクチン接種)が始まるわけではなく、会場の都合で開始時期が遅くなったり、あるいは調査期間の途中で新たな協力者が登録(エントリー)されたりすることもあります。
また、調査の期間中に何らかの理由で脱落する人も必ず出てきます。脱落の理由は、たとえば遠方に引っ越したなど、さまざまありますが、比較的多いのは「副作用がきつかったから」というものです。
その場合、ワクチン群とプラセボ群の間で、脱落者の割合に差が生じてくるはずですが、両グラフはほとんど同じパターンを示しています。つまり、協力者の人数が途中でどんどん減少していっているよう見える(正確に言えば追跡期間の長い人がいなくなっている)のは、脱落が多いためではなく、調査期間の途中で、あるいは終了の間際に多くの協力者が新たにエントリーされていたことを意味します。
いずれにしても、実際の追跡期間はかなり短かったことになります。
「コロナワクチンは打ちませんでした」、「どうして?」、「だって、調査が半年くらいしか行われていなかったから」、・・・・・これは、患者さんなど多くの人たちと、私との間で繰り返し交わされてきた会話です。論文には、「調査は111日間にわたって行なわれた」と記載され、かつ実際の追跡期間はそれより遥かに短かったにもかかわらず、「半年間の調査」という話がなぜか世間で広がっていたのです。
同社の論文には、もうひとつ重大な瑕疵(かし)があったことを指摘しておかなければなりません。ランダム化比較試験でもっとも重要なことは、グループ分けした2群間で、いかなる背景因子にも違いがあってはならないことです。
たとえば、ある評価の高い試験では、年齢や性別はもちろん、既往症、服用中の薬、検査値、運動習慣、食習慣、喫煙の有無、住んでいる地域などから学歴に至るまで数十項目にも及ぶ情報を徹底的に調べ、それらが両群で均等になるようコンピュータで割り振りが行われていました。
それに比べて同社の論文には、背景因子についての記載がなく、FDAに提出された資料に、糖尿病や心不全など8つの病気の有無や人種などの背景因子がかろうじて記されているにすぎませんでした(文献2)。感染するかどうかに重大な影響を与えるのは、居住地や職業、生活習慣などのはずですが、それらが無視されていたのです。
同社は「協力者が多い場合、ランダムに2群に割り付けることで、理論的に背景因子は均等になる」と主張しているのですが、そんな理論は聞いたことがありません。
次回は、製薬企業の、あまりにずさんなデータ管理体制についてまとめます。
【参考文献】
1) Polack FP, et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med, Dec 31, 2020.
2) Pfizer and BioNTech, Vaccines and related biological products advisory committee meeting. FDA Briefing Document, Pfizer-BioNTech COVID-19 Vaccine, Dec 10, 2020.
(2025.11.10)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第7回)?
前回は、製薬企業や多くの専門家がワコロナワクチン有効性の根拠とする「後ろ向き調査」の誤りについて論拠をまとめました。今回は、「製薬企業が発表してきた論文の不正」についてです。
ファイザー社のコロナワクチンに限れば、有効率を求めるためのランダム化比較試験は1つしか行なわれていません(文献1)。
同社は、日本国内でも調査が行われていると主張していますが、それは144人を対象に、中和抗体や短期的な副作用を調べただけのものにすぎません(文献2)。ドイツでも同ワクチンを対象にした調査が行われましたが、対象はわずか60人で、有効率を求めるためのものではありませんでした(文献3,4)。
同社が発表した論文はほかにも数編ありますが、いずれも同じ試験のデータを別の側面から分析したものばかりです(文献5)。
さて、唯一の試験で得られたデータは、次のようなものでした。これらの数値をもとに、同社は「有効率は95%でありWHOが定める最低基準(70%)に比べても十分に高い」と主張しているのです。
論文中に具体的な計算法は明記されていませんでしたが、国内で始まったある訴訟で交わされた同社の書面に、有効率は次のように求めたと記されています。
(1 - (8 / 2.214) / (162 / 2.222)) ×100 = 95.0 (%)
分母は、「対象者が1,000人で観察期間が1年間」となるように換算した値です。両群でほぼ同じ値ですから、単純に表の最上段に示した「対象となった人の数」で割り算しても、ほとんど同じ結果になります。さらに、以下のように簡略化しても同じことです。
(1 - (8 / 162 )) ×100
ここで疑問がいくつかわいてきます。まず、この計算は「感染した人の数」だけで行われていますから、「対象となった人の数」が、たとえば100人でも、あるいは百万人だったとしても同じ結果になってしまうことです。
かりに対象者が百万人だったらどうでしょうか? ワクチンを打って感染を予防できた人が、わずか(162 - 8)人しかいなかったのであれば、副作用による甚大な健康被害だけが残り、集団接種は有害でしかないことになります。
この計算法で得られる値は「相対リスク減少率」と呼ばれますが、実は、ワクチンの有効率を計算する方法が、以下のようにもうひとつあります。
((162/ 18325) - (8 / 18198)) ×100 = 0.84 (%)
この計算法で得られる値は「絶対リスク減少率」と呼ばれ、非常に小さなものとなります。製薬企業としては、当然、前述した相対リスク減少率のほうを公表して、商品をより良く見せたかったわけです。しかし世界保健機関(WHO)は、調査研究の公表に際しては、相対リスク減少率と絶対リスク減少率の両方を提示することが望ましいとしています(文献6; 6頁2段落)。
製薬企業の思惑どおり、医師を含む多くの人々が「ファイザー社のコロナワクチンは95%の人に有効」と、誤解してしまいました。海外の大手メディアもそのことを断罪し(文献7)、また医学専門誌にも、製薬企業のこのような姿勢に対する批判が寄せられています(文献8)。
この論文には、まだまだ疑惑があります。いずれも簡単な内容ではないため、次回以降も、じっくりまとめを行っていくことにします。
【参考文献】
1) Polack FP, et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med, Dec 31, 2020.
2) C4591005試験, 『臨床試験一覧ーPMDA』, pp. 211-229, https://www.pmda.go.jp/drugs/2021/P20210212001/672212000_30300AMX00231_K101_2.pdf
3) C4591005試験, 『臨床試験一覧ーPMDA』, pp. 230-273, https://www.pmda.go.jp/drugs/2021/P20210212001/672212000_30300AMX00231_K101_2.pdf
4) Sahin U, et al., COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature, Sep 30, 2020.
5) Thomas SJ, et al., Safety and efficacy of the BNT172b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med, Sep 15, 2021.
6) Evaluation of COVID-19 vaccine effectiveness in a changing landscape of COVID-19 epidemiology and vaccination. World Health Organization. Oct 1, 2022.
7) Reuter Fact Check, Why relative risk reduction, not absolute risk reduction, is most often used in calculating vaccine efficacy. Reuters, Jun 8, 2021.
8) Olliaro P, et al., COVID-19 vaccine efficacy and effectiveness - the elephant (not) in the room. Lancet, Apr 20, 2021.
(2025.11.03)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第6回)?
前回は、「コロナワクチンは重症化を防いだ」とする専門家の意見は間違っていることを示すデータをまとめました。今回は、専門家が間違いを犯す元になっている「後ろ向き調査の疑問」についてです。
最近、とくにワクチンの有効性を調べる方法として流行となっているのが、PCR検査を受け、結果が「陽性だった人たち」と「陰性だった人たち」を比べるという方法で、テスト陰性調査とも呼ばれています。
まず、この方法で分析した結果を報じた代表的な論文を2編、紹介します。1つ目の調査結果は以下のとおりでした(文献1)。
PCR陽性群 → 4,321人,うちワクチン接種済み, 626人(14.5%)
PCR陰性群 → 37,231人,うちワクチン接種済み,20,520人(55.1%)
以下は2つ目の調査結果です(文献2)。
PCR陽性群 → 182人,うちワクチン接種済み,36人(20%)
PCR陰性群 → 224人,うちワクチン接種済み,83人(37%)
どちらの調査も米国人が対象で、入院患者に限定して行われたものです。背景が共通しているにもかかわらず結果が大きく異なっていて、この分析法の信頼性に疑問のあることがわかります。
このように過去の出来事で比べる分析法は、昔から「症例対照調査」、あるいは広く「後ろ向き調査」と呼ばれてきました。テスト陰性調査も、過去の出来事(ワクチン接種)で比べていますから、後ろ向き調査の変法ということになります。この方法を推奨する専門家は多く、「対象がPCR検査を受けに来た人たちに限定されることから2群間の背景は揃っており、比べることに問題はない」と主張しています。
この主張は正しいでしょうか。次の動画は、症例対照試調査とテスト陰性調査の疑問点をまとめたものです。

英国の政府機関は、後ろ向き調査について、「ワクチン接種を受けていた人たちは、受けていなかった人たちに比べて、背景になにか違いがあったかもしれないので、結果を判定するときには注意を要する」と述べています(文献3,4)。たとえばワクチン接種者には、次のような状況があったかもしれないという意味です。
・普段から健康に気をつけていて、ワクチン接種も積極的だったのではないか
・そのため、PCR検査も積極的に受けに来たのではないか
・接種した安心感から、行動が大胆になり感染リスクが髙かったかもしれない
これらの偏りは、ワクチンの有効率を過大に評価する可能性と、過少に評価する可能性の両面があることになります。同様の指摘は他の研究者からもなされていて、傾聴に値します(文献5)。
テスト陰性調査を含む後ろ向き調査が流行している理由は、コンピュータに記録されているデータを分析するだけで済むことから、予算も人手も要らず、年余におよぶ追跡も必要ないからです。しかも、世界の医学専門誌がこの方法を認め、論文として採用してくれるため、業績をあげたい研究者にとっては「おいしいテーマ」になっているのです。
それだけに、「後ろ向き調査の間違い」が裁判の争点となった場合、手ごわい反論も予想され、さらなる理論構築が必要かもしれません。次回は、「ワクチンメーカー発表論文のデータ操作」についてまとめます。
【参考文献】
1) Thompson MG, et al., Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med, Oct 7, 2021.
2) Tenforde MW, et al., Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≧ 65 years - United States, January-March 2021. MMWR, May 7, 2021.
3) Public Health Scotland COVID-19 & Winter Statistical Report. Feb 16, 2022.
4) Ramsay M, Transparency and data - UKHSA's vaccines report. UK Health Security Agency, Nov 2, 2021.
5) Dean NE, et al., Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med, Oct 7, 2021.
(2025.10.20)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第5回)?
前回は、「コロナワクチンの接種が原因で亡くなった人が確かに増えていた」ことを示すデータをまとめました。今回は、「コロナワクチンは重症化を防いだとする専門家の意見は間違っている」ことを示す論拠についてです。
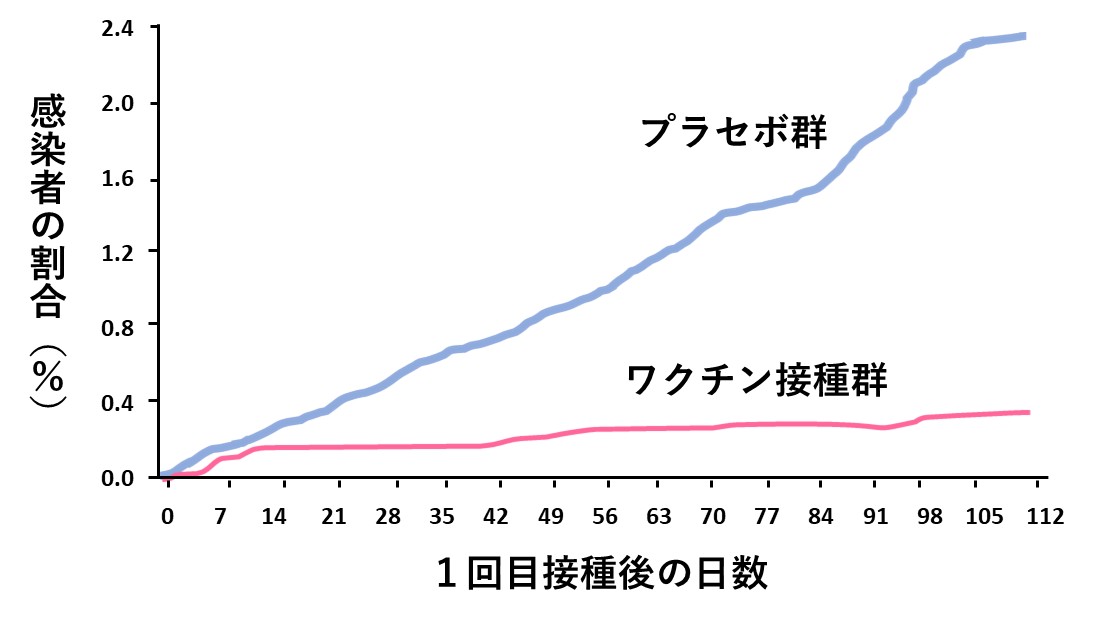
次の表は、ファイザー社がコロナワクチンの有効率(95%)を報じた論文で提示されたデータをまとめたものです(文献1)。本格的なランダム化比較試験の結果であったことから、瞬く間に世界中に知れ渡り、「コロナワクチン有効なり」との世論を形成する元となったのは、ご存じのとおりです。
有効率は、次の表の1行目と2行目の数値から求めたものでしたが、今回、着目するのは3行目の数値(赤字)です。同論文には、「ワクチンを接種した人で重症化したのは1人だけであったことから、重症化を防ぐ効果もあることを示す予備的な(preliminary)エビデンスである」と記されていました。「予備的」との言い訳があったとしても、エビデンスという重い言葉を用いていたことから、「コロナワクチンは重症化予防にも有効」との世論につながっていきました。

次の表の1行目は、重症化を予防する効果を求める正しい計算法と、その結果(赤字)です。「重症化率」で比べると、ワクチン接種を受けた人たちのほうで、むしろ高くなっていることがわかります。
感染した人たちは、(ワクチン接種の有無にかかわらず)免疫力や生活習慣において、感染しなかった人たちと比べて何らかの違いがあったはずです。したがって重症化するか、しないかの違いは、「感染した人たち」に共通する諸要因に伴って成り立つ問題であり、「感染した人の数」を分母にして計算するのは当然のことです。この点は、海外の研究者からも同じ指摘がなされています(文献2)。
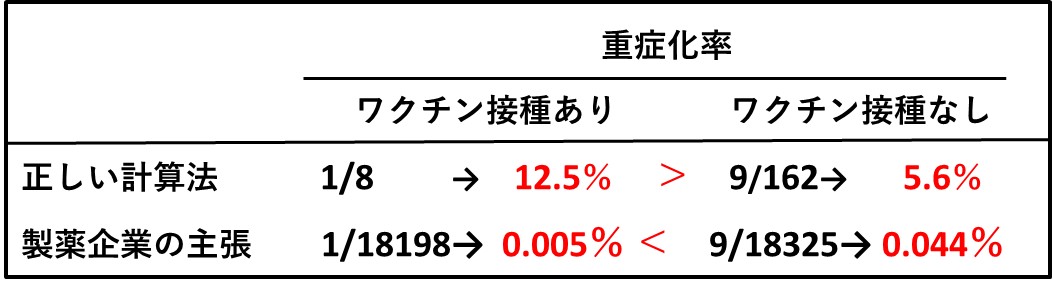
ところがファイザー社は、この指摘に対して、ある裁判で、「重症化予防効果の計算は同表の下段のようにすべき」と主張しているのです(詳細は当ホームページの2024年9月16日付け記事参照)。この計算法によれば、重症化率の大小関係が逆転し、企業側にとって都合のよい結果となります。
さて、2つ目に問題とすべきは、中東アラビア半島に位置する国、カタールで行われた調査の結果についてです。対象は、ファイザー社のワクチンを接種を受けた約百万人。PCR検査を受けて「陽性だった人たち」と「陰性だった人たち」を比べ、「新型コロナ感染の予防効果」と「重症化の予防効果」を計算したものでした。
次のグラフは、その結果です。横軸はワクチン接種を2回受けたあとの月数です。感染を予防する効果は約4ヵ月で半減していますが、重症化を予防する効果は半年ほど続いていたことを示しています。このデータが専門家の間で話題となり、「感染を防ぐ効果は弱いが、重症化を予防する効果はあり」という、神話にも似た言説の根拠のひとつになったのです。
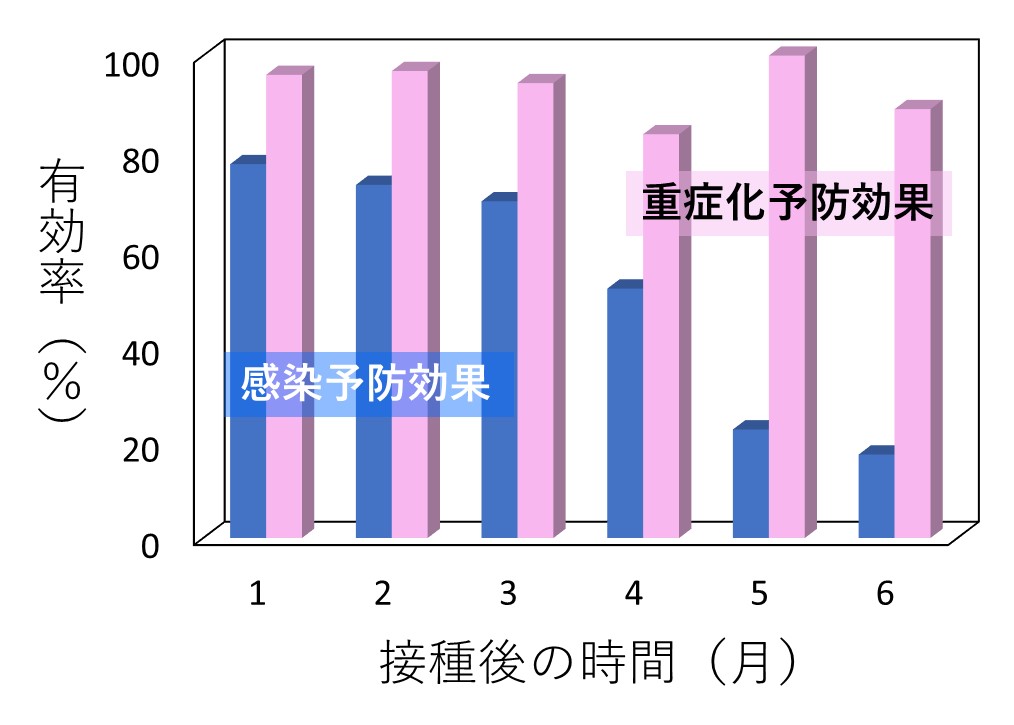
この調査は、ランダム化比較試験とはまったく異なる、いわゆる「後ろ向き試験」で行なわれたものでした。この方法では、比べる2つのグループの背景をそろえることができず、結果はまったく信頼できないものとなります。実際、感染を予防する効果がなくなったあとも、重症化だけは防ぐことができるとの主張には、あきらかな矛盾があります。
次回は、ワクチン問題の根幹をなす「後ろ向き調査の欠陥」について復習することにします。
【参考文献】
1) Polack FP, et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med, Dec 31, 2020.
2) Wang X, To the Editor, "Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med, Apr 22, 2021.
3) Thomas SJ, et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med, Sep 15, 2021.
4) Chemaitelly H, et al., Waning of BNT162b2 protection against SARS-CoV-2 infection in Qatar. N Engl J Med, Oct 6, 2021.
(2025.10.20)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第4回)?
前回は、「なぜコロナワクチンを接種すると感染率が高まってしまうのか」という問題について、そのメカニズムを説明する論拠を取り上げました。第4回目の今回は、「コロナワクチンの接種が原因で死亡した人が増えていた」ことを示す確たるデータをまとめます。
コロナワクチン接種を受けたのち死亡したケースについて、病理解剖を行って死因を分析したという報告が多数あります。米国のある研究者は、678件もの関連論文を精査。PRISMAと呼ばれる評価基準に従ってデータを厳選し、集計しています(文献1)。
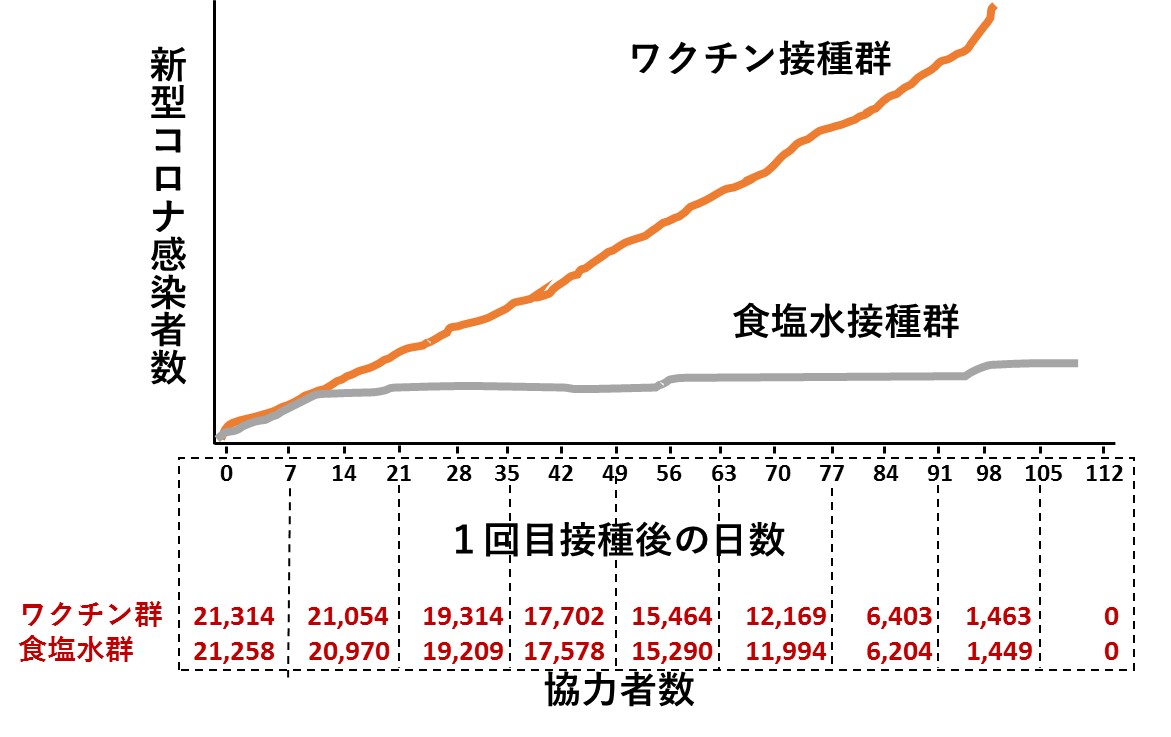
次の左側のグラフは、そのうち「ワクチン接種後の経過日数と死亡例の人数」をまとめたものです(論文に掲載された図を私がイラストで再現したもの)。
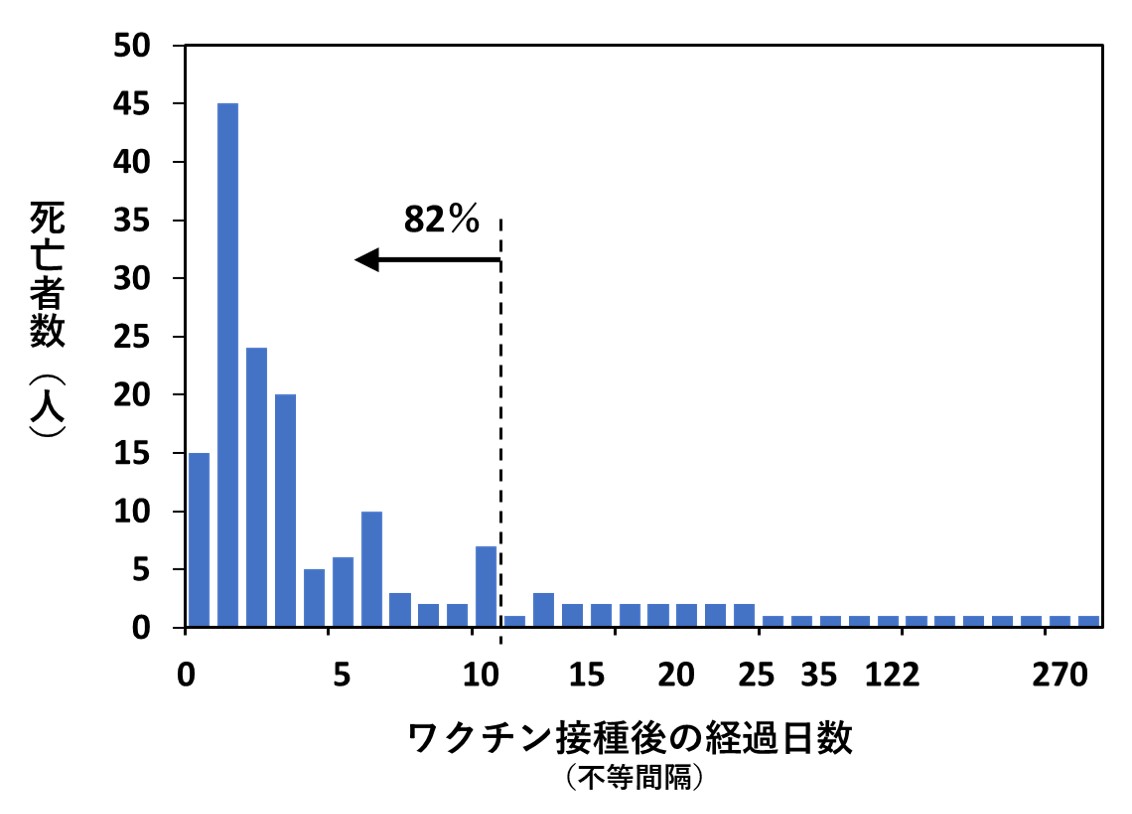
また上の右側のグラフは、厚生労働省が公表しているコロナワクチン接種後死亡データのうち、令和3年2月17日~令和4年4月17日の間(1年2ヵ月)に報告がなされた1,609例(文献2)を、私がグラフにしたものです。横軸は、左側のグラフと同じ「ワクチン接種後の経過日数」です。
異なる国で、異なる基準で集計されたデータであるにもかかわらず、2つのグラフは非常によく似ています。報告例の大部分がワクチン接種後10日以内の死亡であった点も共通しています。
これらのデータは、「特定の原因が特定の結果をもたらしているかどうかを検証するための条件」として知られるブラッドフォード・ヒルの8条件(文献3)を満たしていることから、因果関係はあきらかです。
次のグラフは、「2021年1年間の月別のワクチン接種後死亡報告例」のデータ(文献2)に、「同じ年のコロナワクチン接種者数」のデータ(文献4)を重ねたものです。ワクチン接種者数(赤色)は、死亡者数(青色)に比べて絶対数が圧倒的に多いため、適当に圧縮してあります。両者の正しい値は、左右の目盛でご確認ください。点線で囲った部位に着目すると、両者の間に強い相関関係のあることがわかります。
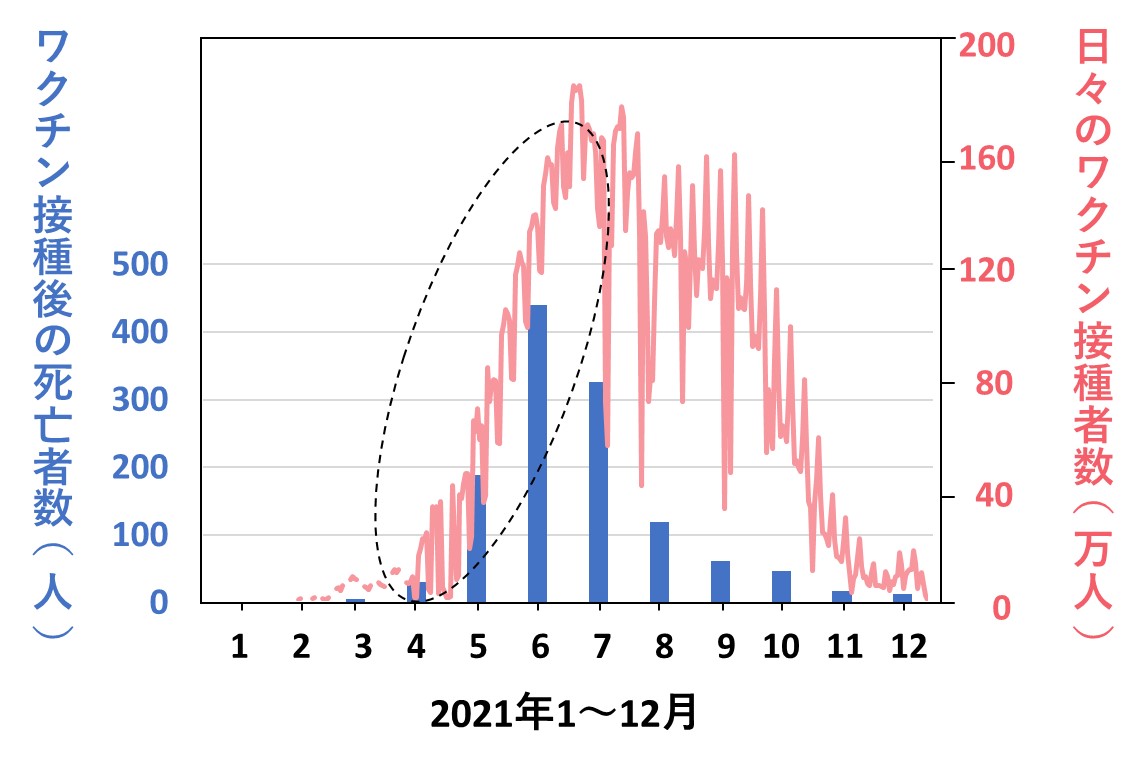
最後のグラフは、2021年2月21日~2023年12月21日における国内での「コロナワクチン接種数」(青色)と「総死亡者数」(赤色)の週ごとのデータを重ねて表示したものです。縦軸の「死亡者数」は、ワクチンと無関係に(原因を問わず)日本人全体で亡くなった人数(総死亡数)となっています。
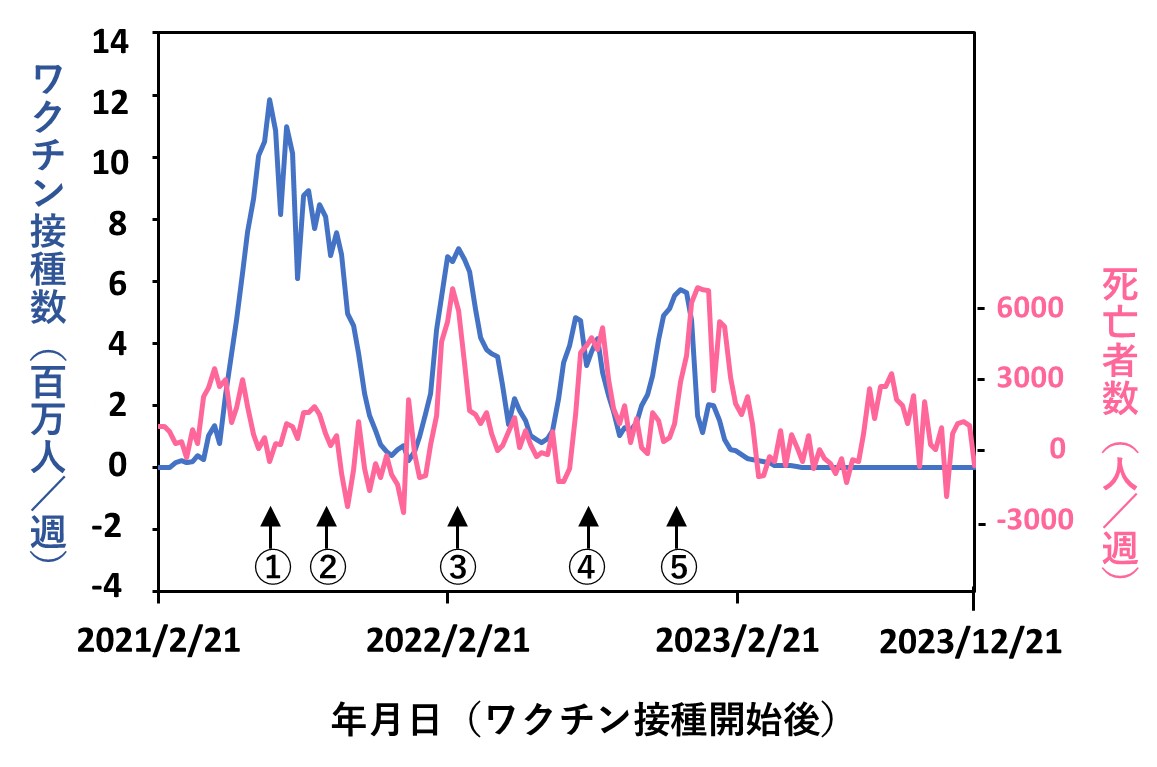
両データの出典はそれぞれ文献4と5です。そのうち総死亡数は、実際の人数でなく、季節変動や経年の自然増を数学的処理で補正した値となっています(詳細は当ホームページの2024年7月15日、22日付け記事を参照)。また2023年3月以降のワクチン接種数のグラフが平坦になっているのは、行政のデータ公表が終了したためであり、接種が行われなくなったわけではありません。
図中①~⑤は、国の方針に従ってワクチン接種が繰り返された回数を示しています。3回目以降は、ワクチン接種件数のピークを追うように総死亡数のピークが認められています。(1回目と2回目は、接種の呼びかけが連呼され、人々が接種会場に殺到した様子がわかりますが、総死亡数にピークが認められない理由は不明です。アイデアがあればご意見をお寄せください)
次回は、「コロナワクチンは、少なくとも重症化を防いだ」と主張する専門家の誤りを正すデータをまとめます。
【備考】 分析に利用したEXCELァイルについて
「コロナワクチン接種後死亡例」」として厚生労働省が公表しているデータはPDFファイルのため、直接、読み取ることができません。そこで、私がエクセルファイルに変換し、欠損データを削除するなどして修正を施したものをグラフの作成に使用しました。文献6をクリックすると、そのファイルをダウンロードすることができます。
厚生労働省のホームページには、「データの利用は自由であり、加工を行った場合でも、その旨を明記すれば許諾を求める必要はない」と記されています。したがって同ファイルについて著作権上の問題はありませんが、ネット上にグラフなど公開する場合は、引用元を明記してください。
【参考文献】
1) Hulscher N, et al., A systematic review of autopsy findings in deaths after COVID-19 vaccination. Science, Public Health Policy, and the Law, Nov, 2024.
2) 第80回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第5回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)資料, https://www.mhlw.go.jp/content/10601000/000948953.pdf, accessed: Oct 15, 2025.
3) Redert A, Causal effect of covid vaccination on mortality in Europe. ResearchGate, Feb 24, 2023.
4) Coronavirus (COVID-19) vaccinations. Our World in Data, Apr 19, 2023.
5) 国立感染症研究所 感染症疫学センター, https://exdeaths-japan.org/graph/weekly_cause, accessed: Oct 15, 2025.
6) エクセルファイルmortalityinterval.xlsx へのリンク
(2025.10.13)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第3回)?
前回は、「コロナワクチンを接種すると、むしろ感染しやすくなってしまう」ことを示すエビデンスを整理しました。今回は、それがなぜなのかをまとめます。
まず、コロナワクチン接種によって体内で作られる抗体は、IgG(アイ・ジー・ジー)というたんぱく質でできています。IgGには、IgG1~IgG4の4つの種類があり、それぞれ役割が異なっています。最近の研究から、とくにIgG4が、ワクチンの副作用を考える上で重要であることがわかってきました。
IgG4は、本来、免疫反応が過剰にならないよう、ほどほどにブレーキをかける役割を担うものです。しかし強すぎるワクチンのせいで、ウイルスを無毒化する反応まで止めてしまうのです(文献1)。
次の左側のグラフは、ボランティアを対象に、ファイザー社のワクチンをそれぞれ2回目、3回目の接種を行った10日後、血液中のIgG1~IgG4を測定した結果です(文献1)。グラフの縦軸は、2回目接種後に対する3回目接種後の「各測定値の増加率」を表しています。
同じく右側のグラフは、「IgG全体に対するIgG4の割合(%)」の経時変化を示したものです。両グラフから、ワクチン接種の回数が増えるごとに、また接種後の時間が経つにつれて、免疫機能にブレーキがかかっていくことがわかります。
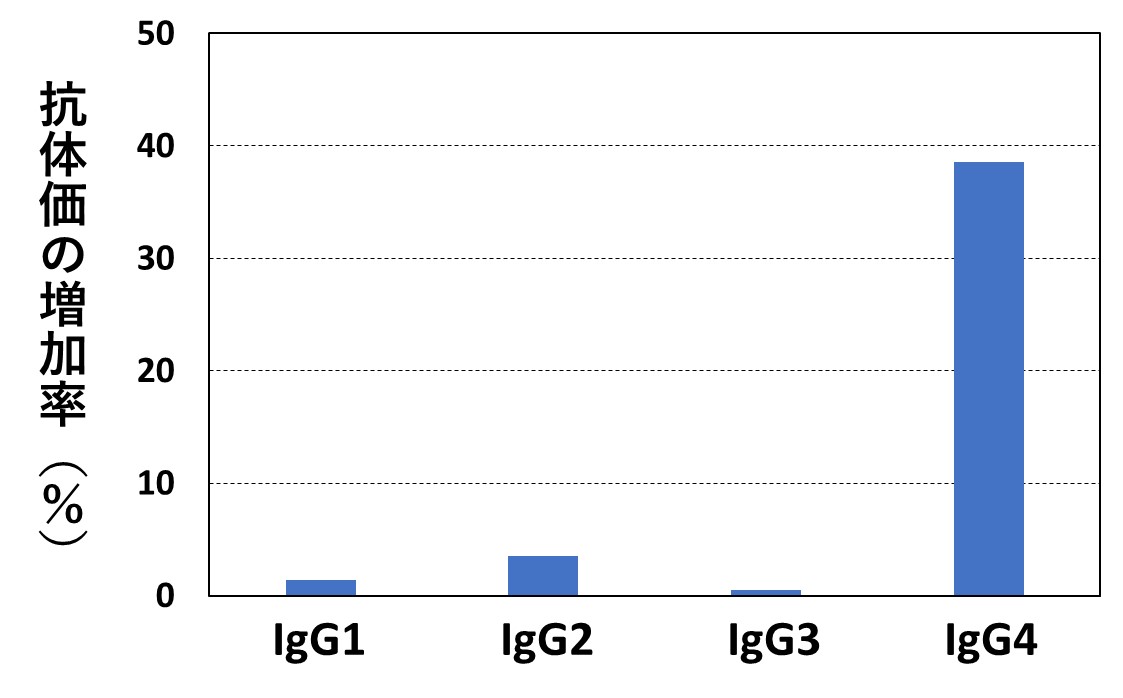
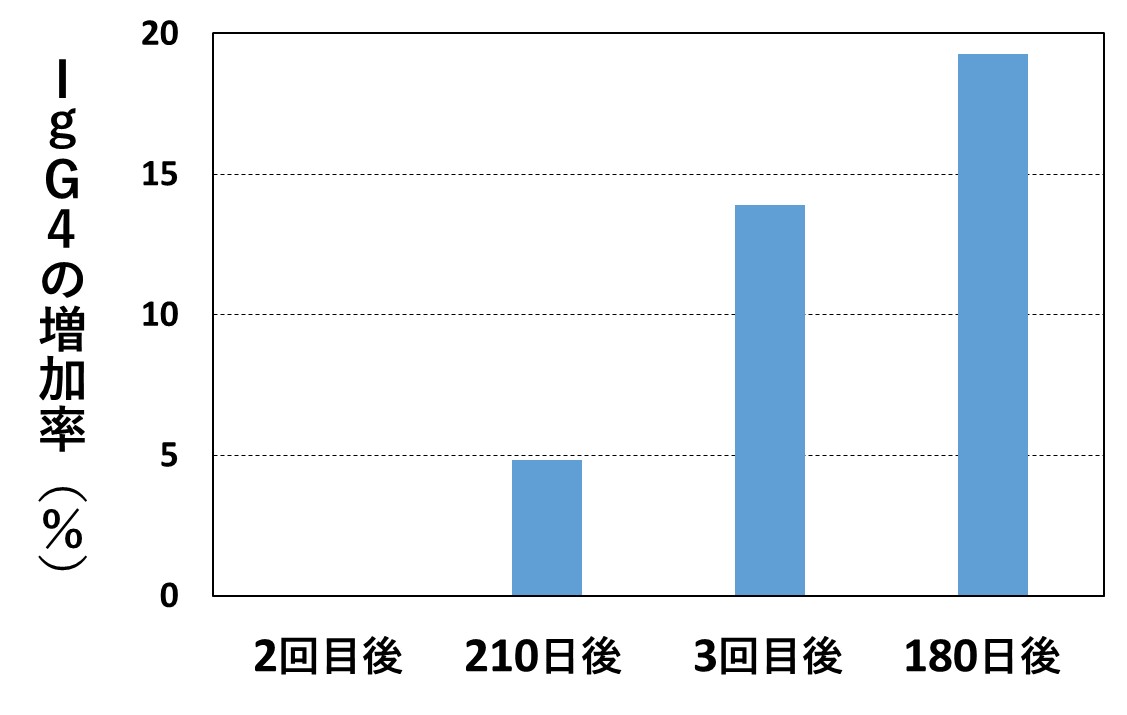
コロナワクチンを繰り返し接種すると、IgG全体の産生も低下してしまうことを示したデータも発表されていて(文献2)、感染率が高まるメカニズムとして説得力があります。(詳細は、当ホームページの2024年2月26日記事の(2)参照)
コロナワクチンの接種によって、感染しやすくなってしまう理由が、もうひとつあります。まず、以下のような事実が以前から指摘されていました。
・2009年に流行したインフルエンザ(H1N1型)に感染して死亡した人の
多くは、以前、別のインフルエンザ(H2N2型)が世界的に大流行した
時期に生まれていた(文献3)
・抗原の種類を増やした新しい「9価の子宮頸がんワクチン」は、以前から
使われていた5価ワクチンをすでに接種している人に打っても効かない
(文献4)
・コロナウイルスは夏カゼの原因として以前から存在していたが、それに感染
したことがある人のうち、約2割は新型コロナウイルスに対する中和抗体が
できない(文献4)
これらの事実をふまえて、以下の動画をご覧ください。

「抗原原罪理論」は、研究がまだ始まったばかりで、詳細が解明されているわけではりませんが、その存在を疑う余地はありません(文献4~7)。もし、このような仕組みがなければ、1種類の抗原に対して、似て非なる抗体がたくさんできてしまい、自己免疫病のような危険な免疫反応が体内のあちらこちらで起こってしまうことになります。
とくに新型コロナウイルスは突然変異を起こしやすく、似て非なる変異株がどんどん出現してきました。そのため、抗原原罪理論に従って中和抗体の産生がブロックされ、結果的に「ワクチンを接種したほうが感染しやすくなってしまう」という、従来の免疫学の常識を覆すような現象が起こっているのです。
次回は、コロナワクチンで死亡者が急増したことを示すデータを取り上げます。
【参考文献】
1) Irrgang P, et al., Class swithch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci Immunol, Jan 27, 2023.
2) Gao F-X, et al., Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. iScience, Dec 22, 2022.
3) Gagnon A, et al., Pandemic paradox: early life H2N2 pandemic influenza infection enhanced susceptibility to death during the 2009 H1N1 pandemic. mBio, Jan 16, 2018.
4) Brown EL, et al., Original antigenic sin: the downside of immunological memory and implications for COVID-19. mSphere, Mar 10, 2021.
5) Christopher T, et al., What are the primary limitations in B-cell affinity maturation, and how much affinity maturation can we drive with vaccination? Cold Spring Harb Perspect Biol, May 1, 2018.
6) Monto AS, et al., The doctrine of original antigenic sin: separating good from evil. J Infect Dis, Jun 15, 2017.
7) Pusnik J, et al., Vaccination impairs de novo immune response to omicron breakthrouth infection, a precondition for the original antigenic sin. Nat Commune, Apr 10, 2024.
(2025.10.6)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第2回)?
『確固たるエビデンス中間まとめ』の第2回は、「コロナワクチンを接種した人たち」のほうが「接種しなかった人たち」に比べ、より感染しやすかったことを示すデータについてです。
最初のデータは、世界68ヵ国を対象に、国民のワクチン接種率と新規感染者数との関係を調べたものです(文献1)。次のグラフに示すとおり、接種率が高い国ほど感染者数があきらかに多くなっています。調査は、デルタ株が優勢だったころに行われました。
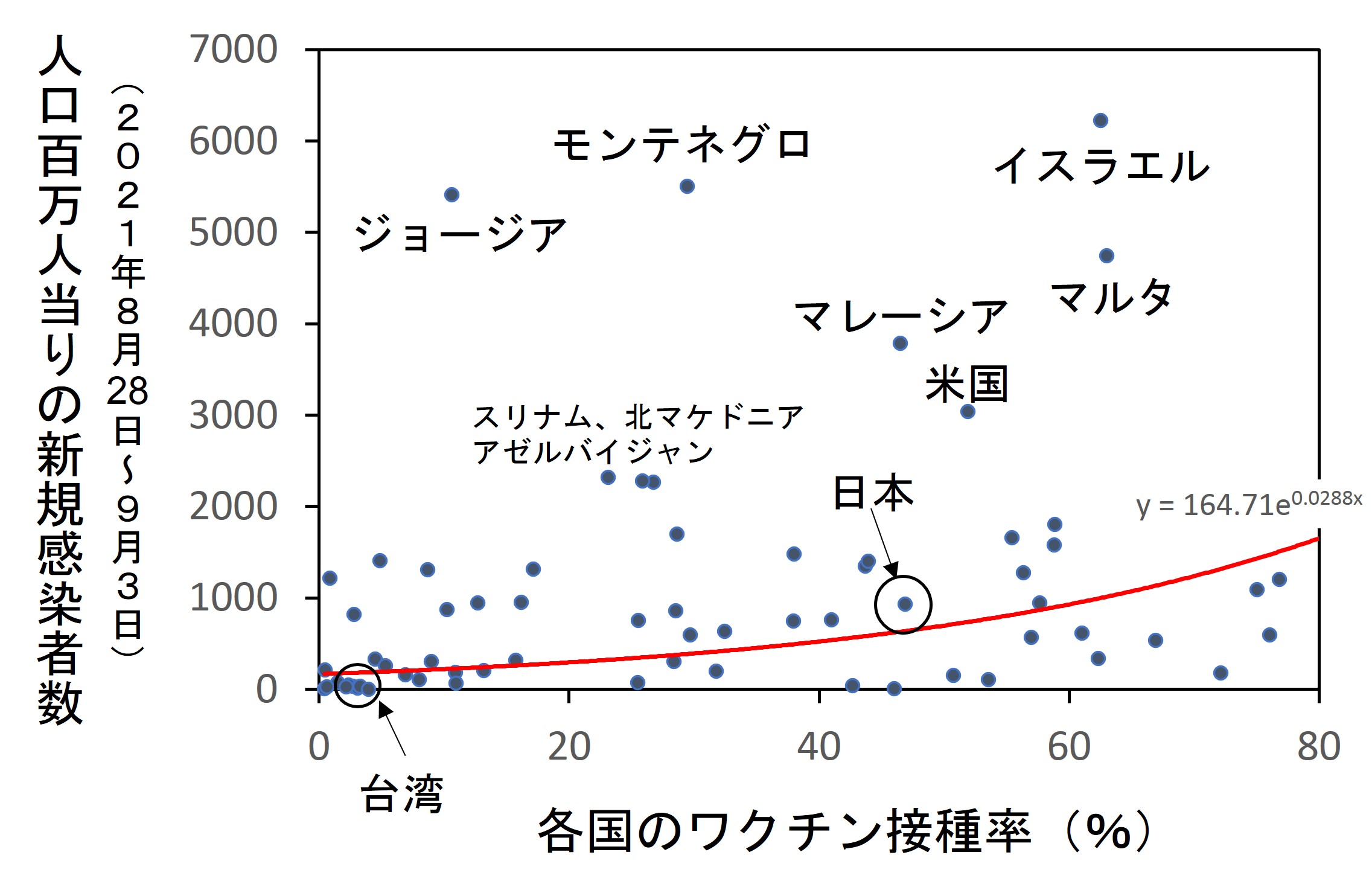
同じ現象はほかの多くの調査でも確認されていて、とくに英国の政府機関が発表したデータが明快です(文献2)。詳細は2024年2月26日付け記事の(2)を参照してください。
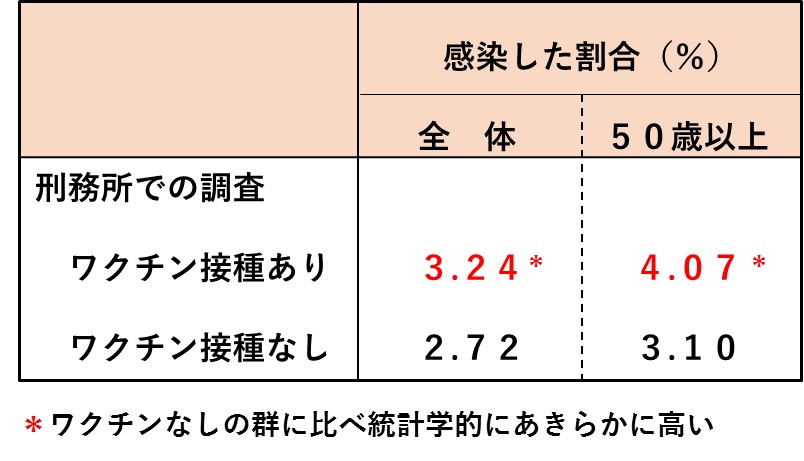
次の表は、米国カリフォルニア州の33の刑務所で、受刑者を対象に、ワクチン接種の有無で新型コロナの感染割合に違いがあるかを調べた結果です(文献3)。対象は9万6千人で、従来株とオミクロン株の抗原を合わせた2価ワクチンが使われていた時期の調査です。ランダム化比較試験ではありませんでしたが、生活環境や日常行為がほぼ同じ人たちを対象にしたものですから、説得力があります。(詳細は2024年11月11日付け記事参照)
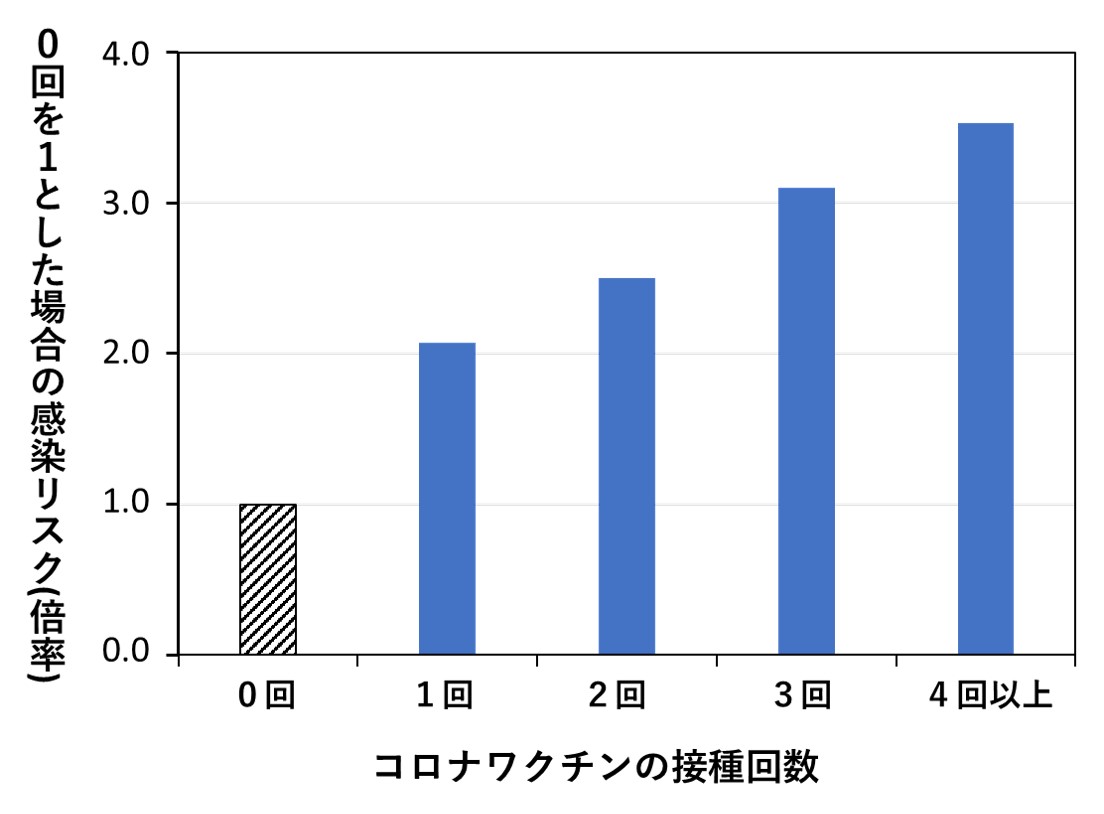
次のグラフは、米国オハイオ州に本拠をおく、ある医療グループの従業員4万8千人を対象に、コロナワクチンを接種した回数とその後に感染した割合を調べた結果です(文献4)。年齢、性別、職種、患者に接する業務か否かなど、感染に影響を及ぼす諸要因が統計処理によって補正されており、接種回数が多い人ほど感染リスクが高かったことを、明確に示す結果となっています。(詳細は2024年11月11日付け記事参照)
最後のグラフは、ワクチン接種後の中和抗体の測定値(力価)とワクチンの有効率との関係を調べた多数の研究報告をまとめたものです(文献5)。図中、丸印の大きさは、各研究で調査対象となった人数の多さを反映しています。中和抗体の値はワクチンの効果を表すものでないことがわかります。
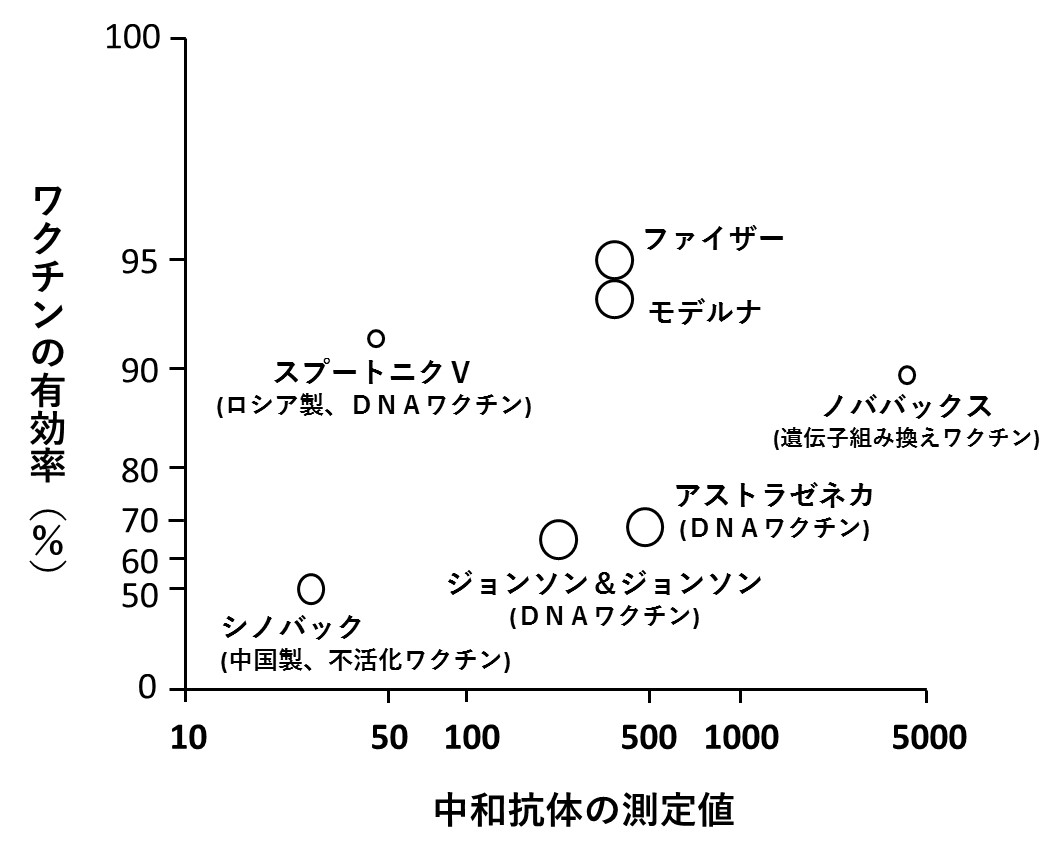
ワクチンメーカーが主導した多くの臨床試験で、「中和抗体さえ上がればワクチンは有効だ」と、決めつけていることに対する反論として、説得力のあるデータとなっています。(詳細は2024年10月21日付け記事参照)
以上が、コロナワクチンが免疫力をむしろ低下させてしまうことを示すエビデンスです。次回は、なぜ免疫機能に悪影響を与えるのかについて、まとめます。
【参考文献】
1) Subramanian SV, et al., Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. Eur J Epidemiol, Sep 30, 2021.
2) Public Health Scotland COVID-19 and Winter Statistical Report, as at 17 January 2022, Public Health Scotland, Jan 19, 2022. at https://medicalmafia.s3.us-west-1.amazonaws.com/page_article/22-01-19-covid19-winter_publication_report.pdf, page 38.
3) Ko L, et al., COVID-19 infection rates vaccinated and unvaccinated inmates: a retrospective cohort study. Cureus, Sep 4, 2023.
4) Shrestha NK, et al., Effectiveness of the coronavirus disease 2019 bivalent vaccine. Open Forum Infect Dis, Apr 19, 2023.
5) Earle KA, et al., Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine, Jul 22, 2021.
(2025.9.29)
Q&A 反論を許さない確固たるエビデンスとは(中間まとめ 第1回)?
当ホームページの情報量が膨大となり、以前の記事を検索するのも大変になってきました。そこで今回から、しばらくの間、過去の記事の中から、選りすぐりのエビデンスをまとめることにしました。とくに、コロナワクチンによる健康被害を裁判という手段で訴える人も多くなってきましたので、法廷での論争で負けないための資料になれば、幸いです。
mRNAタイプのコロナワクチンを接種したあと、長期にわたり体調を崩している人が少なくありませんが、接種後、しばらく経ってから認められた症状や病気に対しては、「因果関係なし」という判定が下されてしまうようです。そこで今回は、中間まとめの第一弾として、「ワクチンの副作用と診断された報告事例で、接種からどれくらい経って発症しているのか」という問題に焦点をあてます。
以下はその一覧ですが、すでに当ホームページで紹介した内容もあれば、今回、新たに収集したデータも含まれています。
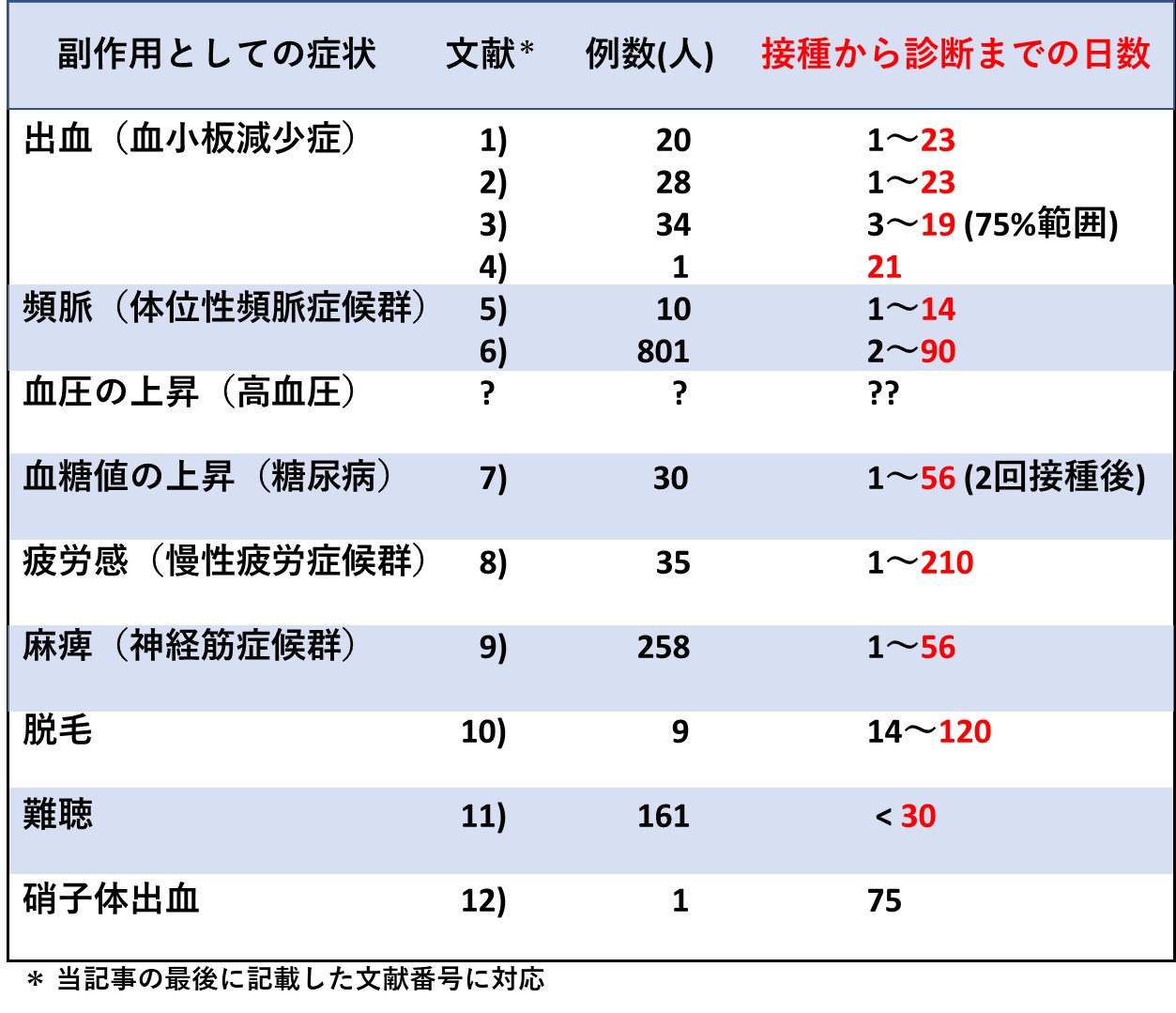
同表からわかるように、症状によっては接種後、100日以上も経ってから診断された事例も報告されています。なお、「血圧が上がったままさがらない」と悩んでいる人もいますが、接種後の血圧変動を長期にわたって調べたというデータがいまのところ見つかっていません。
各データの引用元は、いずれも医学専門誌であり、SNSなど不確かな情報源のものは取り上げていません。文献8)は、いわゆる査読なしのWebジャーナルに掲載されたものですが、査読者(審査員)のつもりで私が精読し、正当な内容と判断しました。
同論文の著者は米国イェール大学の研究グループで、指導者の岩崎明子教授には、数々の受賞歴があります。新型コロナウイルス感染症の後遺症に関する研究で2025年の慶應医学賞も決まり、国内でも報じられたところです。コロナワクチンの副作用についても基礎研究を進めていて、今後の成果が期待されます。
各症状や病気のメカニズムについては、当ホームページ中にそれぞれ解説があります。Microsoft EdgeやYahoo!JAPANでご覧の方は、画面上段の右端にある「・・・」マークをクリックし、「ページ内の検索」を選択することにより、任意のキーワードで検索することができます。
次回以降も、テーマ別にエビデンスのまとめを行っていく予定です。
【参考文献】
1) Lee E-J, et al., Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am j Hematol Mar 9, 2021.
2) Welsh KJ, et al., Thrombocytopenia including immune thrombocytepenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine, Jun 8, 2021.
3) Jiang D, et al., Platelet trends after Covid-19 vaccination in patients with chronic or persistent immune thrombocytopenia. Am J Hematol, Dec 1, 2021.
4) Prasad S, et al., Immune thrombocytopenia following COVID-19 vaccine. Case Rep Hematol, Jun 25, 2022.
5) Teodorescu DL, et al., Postural orthostatic tachycardia syndrome after COVID-19 vaccination. Heart Rhythm, Jan, 2023.
6) Bushi G, et al., Postural orthostaic tachycardia syndrome after COVID-19 vaccination: a systemic review. BMC Cardiovasc Disord, Nov 13, 2024.
7) He Y-F, et al., Correlation between COVID-19 vaccination and diabetes mellitus: a systemic review. World J Diabetes, Jun 15, 2023.
8) Bhattacharjee B, et al., Immunological and antigenic signatures associated with chronic illness after COVID-19 vaccination. medRxiv, Feb 18, 2025.
9) Tayebi A, et al., Neuromuscular diseases associated with COVID-19 vaccines: a systemic review and pooled analysis of 258 patients. BMC Neurol, Dec 11, 2023.
10) Scollan ME, et al., Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep, Feb, 2022.
11) Fisher R, et al., The association between COVID-19 vaccination and idiopathic sudden sensorineural hearing loss, clinical manifestation and outcomes. Eur Arch Otorhinolaryngol, Feb 17, 2023.
12) Matsuo T, et al., Temporal association of vitreous hemorrhage and hypertension after COVID-19 mRNA vaccines. Clin Case Rep, Nov 27, 2022.
(2025.9.22)
Q&将来のパンデミックに備える?
前々回から、子供に感染させないことが感染症の大流行(パンデミック)を防ぐポイントになること、および学校閉鎖には効果があまり期待できず、不利益も大きいかもしれないことを記してきました。今回は、ではどうすればいいのかを考えます。
新型コロナ感染症のパンデミックが始まったばかりのころ、「マスク」、「手洗い」、「ソーシャルディタンス」という言葉が盛んに使われましたが、実は、100年以上前に発生したスペイン風邪の大流行の際に作られた言葉でした。その効果については異論もありましたが、対策がこれだけしかなかった時代、大惨事が3年ほどで終息したこともまた事実です(文献1)。やはり、この3つが守るべき大原則でしょう。
日本では、新型コロナが広がり始めたころ、医師の間で不思議なウワサが広まっていました。「ステロイドホルモン入りの点鼻液を毎日使っていると、コロナにかかりにくい」というものです。そのせいで、全国の薬問屋さんの倉庫は、その薬だけ品切れになっていた、というもっともらしい話題もついて回っていました。同僚の医師たちも行っていたようでしたが、この話が、その後、どうなったのかはわかりません。
さて、今回の話題は、昔から花粉症などに使われてきた「普通の点鼻液」に、新型コロナの感染を防ぐ効果があると結論した研究発表についてです(文献2)。
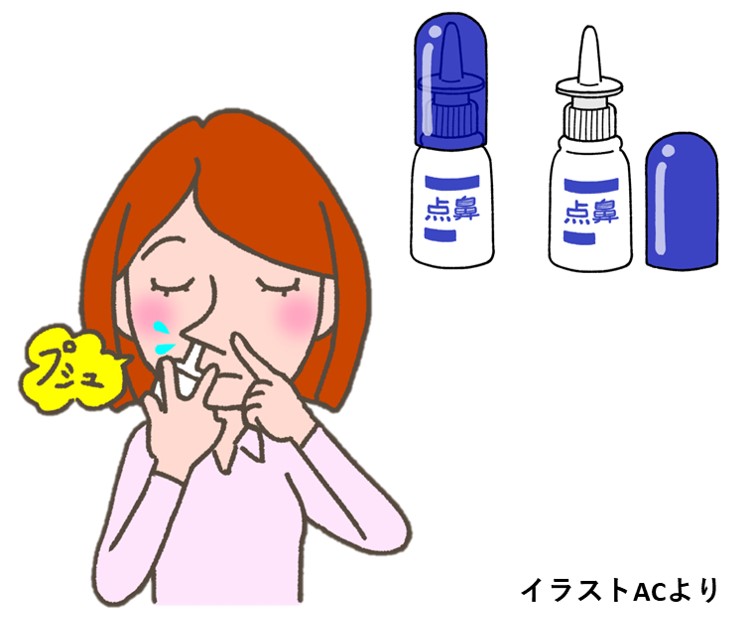
その研究では、まず健康な成人560人を無作為に2つのグループにわけ、一方にアゼラスチンという抗ヒスタミン薬を(以下、実薬)、他方にはプラセボを配合した点鼻液を、左右の鼻腔に1日3回ずつ、平均して56日間、噴霧してもらいました。この間、参加者は専任のスタッフから、週2回ずつ鼻腔ぬぐい液の抗原検査を受け、陽性となった人にはPCR検査も施行されました。
56日間の観察期間中、新型コロナに感染した人は、実薬群で227人中5人(2.2%)、またプラセボ群では223人中15人(6.7%)でした。発熱などあきらかな症状があった人に限ると、その差はさらに大きなものになっていました。
この研究が優れていた点は、新型コロナ以外にも、インフルエンザウイルス、アデノウイルスなど20種類ほどの感染性病原体についても検査が行われていたことです。結果的に、これらさまざまな病原体の検出率も、実薬群のほうで少なかったとのことです。
副作用については、訴えの総数に差がなく、鼻出血や苦み(にがみ)を感じるなどの症状が実薬群のほうでやや多くなっていました。
以上、良いことずくめの話ですが、気になる点もいくつかあります。調査の対象人数が少なすぎること、鼻腔噴霧を毎日3回、長期間にわたって続けなればならないこと、そしてなぜ有効なのか、そのメカニズムがよくわからないことです。幸い、大手製薬企業とのつながりはなさそうです。
この点鼻液の主成分アゼラスチンは、第二世代の抗ヒスタミン剤に分類され、アレルギー反応の元になるヒスタミンやロイコトリエンの放出を抑え、同時にH1受容体と呼ばれる部位をブロックして、ヒスタミンが働かないようにする働きをします。日本に同じ製品はありませんが、同系統の薬が配合された点鼻液が国内でも販売されています。しかし、それらの点鼻液に同じ効能があるかどうかは不明です。
感染症の流行は長期にわたって続くものですから、その予防のために何らかの薬を漫然と使い続けるのは、副作用のリスクを高めることになり、本末転倒になりかねません。パンデミックの対策は、一朝一夕に成し遂げることができず、地道な研究を積み重ねていくしかなさそうです。
【参考文献】
1) Berche P, The Spanish flu. Presse Med, Jun 1, 2022.
2) Lehr T, et al., Azelastine nasal spray for prevention of SARS-CoV-2 infections, a pahse 2 randomized clincal trial. JAMA Intern Med, Sep 2, 2025.
(2025.9.15)
Q&A 子供たちを感染させないために必要なこと?
前回は、「間違いだらけのワクチン効果を報じた論文」を絶賛した有名専門誌のコメント記事を紹介しました(文献1)。その中に、もうひとつ気になる記述がありました。「学校閉鎖は効果がなく、むしろ有害」との見解で、将来のパンデミック対策を考える上で重要なテーマですから、今回はその真偽を考えます。
日本でも、総理大臣が、全国の小学校、中学、高校を2020年3月2日(月)から閉鎖する、と唐突に宣言したのをご記憶と思います。当時、そのことについて賛否ありましたが、多くは感情論的な意見でしかありませんでした。しかし、大勢の健康な子供たちを相手に、ランダム化比較試験を行うわけにもいかず、その評価は簡単でありません。
同じころ、欧米でも学校閉鎖がなされ、効果を分析した研究発表もいくつかありました。しかし、どの国でも都市封鎖が同時に行われていたため、学校閉鎖の効果だけを評価するのは難しく、そのため結論もはっきりしないものばかりでした(文献2)。
日本は、都市封鎖を行なわなかった、世界でもまれな国のひとつでした。当時、この点に着目した2つの研究が、日本国内で行われ、いまも光を放つ存在となっています(文献2,3)。ひとつは、神戸の研究者たちが行った研究で、「学校閉鎖を行っても新規感染者の人数は減っていなかった」ことを証明したものです(文献2)。
研究では、まず学校封鎖が要請された日の「60日前」から「15日後」までの間の新規感染者数を公表データから調べ、計算式を組み立て、学校閉鎖後の数値を予測しました。もし実際の感染者数が、予測した値よりあきらかに少なくなっていれば、学校の閉鎖が地域全体の感染予防に役立っていたことになります。
次の図は、論文に掲載された「学校閉鎖の前後における新規感染者数の推移グラフ」を元に私が描いたイラストです。閉鎖の要請は月曜日からでしたが、実質的には前の週の土曜日2月29日が開始日でした。
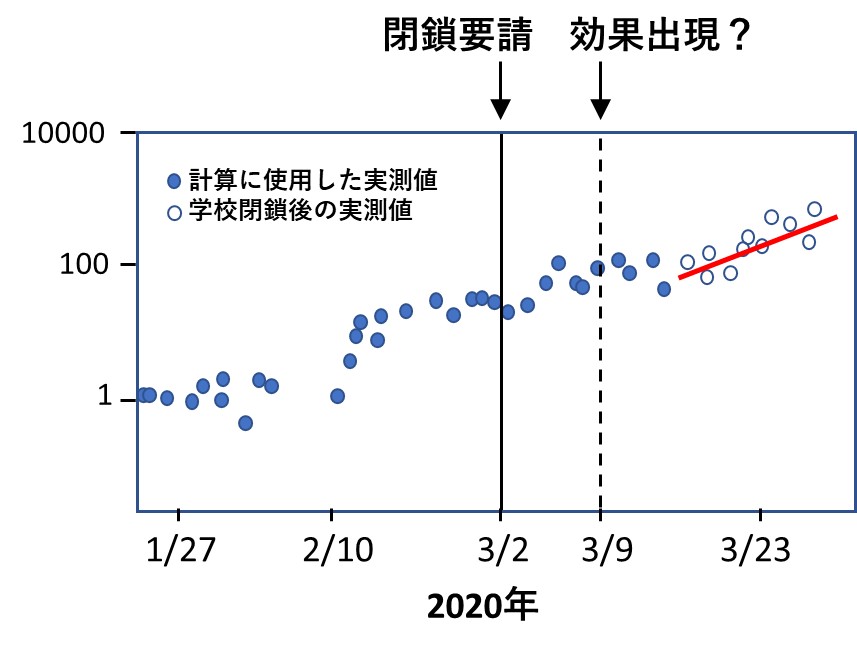
新型コロナ感染症の潜伏期は最長で5日、また当時の国の方針でPCR検査は発症後4日以内の人に限定されていましたから、感染者数に変化が現れるとしても9日後になる、との想定で計算が行われました。図中、●印は実際の感染者数で、赤い実線が学校閉鎖後の予測ですが、それより増えてもいなければ、減ってもいなかったことがわかります。
もうひとつの研究は、一橋大学からの発表でした(文献4)。学校閉鎖の要請が出たのは、ちょうど春休みに入る直前で、その後3ヵ月ほど続きましたから、小学校入学の時期と重なっていたことになります。つまり、入学年齢に達する前後の年齢の子供たちに注目すると、その4月に入学した子供は学校閉鎖に遭遇し、月数がまだ満たない子供は、幼稚園や保育園に通い続けていたことになります。
具体的には、生後53ヵ月(4歳5ヵ月)~124ヵ月(10歳4ヵ月)の子供たちとその両親に協力を求めアンケート調査が行われました。質問項目は、学校閉鎖で「子供の体重が増えたと思いますか?」、「子供とどう接すればよいか悩みましたか?」などでした。その結果がグラフとして論文に掲載されていますが、以下は、そのひとつを私がイメージ図として描いたものです。
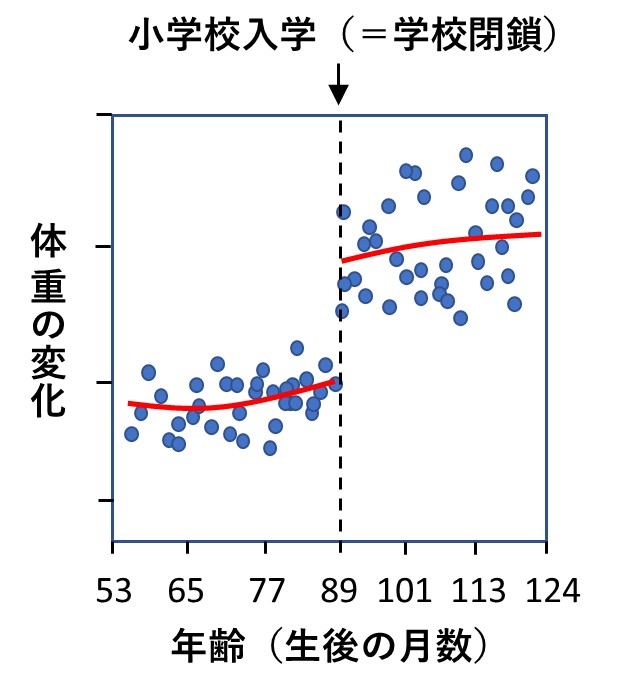
育ち盛りの子供たちですから、短期間でも体重は自然に増えていきますが、学校が閉鎖された途端、あきらかな増加が認められたのです。毎日、歩いて学校に通うこと、規則正しい生活を送りながら栄養バランスのとれた給食をとることなどが、子供たちの健全な成長にとって大切だと、研究者たちはコメントしています。
学校は、単に知識を与えるだけの場ではなく、子供たちの健康を育む上でも重要だというわけです。また海外の研究では、長期間の学校閉鎖を経験した人たちは、将来の収入があきらかに低くなるとも指摘されています(文献5)。(その他の質問項目の結果は省略します)
この研究のポイントは、比較する2群の年齢が非常に近く(誕生日の違いが1年未満)、学区がいっしょで生活環境なども共通していることから、ランダム化比較試験に求められる要件がほぼ満たされていたことです。
この2つの研究によってわかったのは、「学校閉鎖には地域全体の感染拡大を防ぐ効果がなく、かつ子供たちに健康上の不利益をもたらす可能性がある」ということでした。したがって今後は、学校閉鎖以外の方法で、パンデミックを防ぐ方策を考えなければならないことになります。
【参考文献】
1) Gandhi M, COVID-19 vaccination saved lives and this matters in 2025. JAMA Health Forum, Jul 25, 2025.
2) Auger KA, et al., Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA, Jul 29, 2020.
3) Iwata K, et al., Was school closure effective in mitigating coronavirus disease 2019 (COVID-19)? time series analysis using Bayesian inference. Int J Infect Dis 99: 57-61, 2020.
4) Takaku R, et al., What the COVID-19 school closure left in its wake: evidence from a regression discontinuity anaslysis in Japan. J Public Econ, Jan 8, 2021.
5) Donohue JM, et al., COVID-19 and school closures. JAMA, Sep 1, 2020.
(2025.9.8)
Q&A 憂うつな医学専門誌の”思い込み”?
前回は、有名医学専門誌が報じた「間違いだらけのワクチン効果」を取り上げて検討しました(文献1)。ところが、その論文を「すばらしい研究」と大絶賛したコメント記事が同じ号に掲載され、あたかも同誌の公式見解であるかのように扱われていたのです(文献2)。
今回は、そのコメント記事を紹介し、コロナワクチン問題に対する専門家たちが陥った誤謬を考えます。論旨は以下のようなものでした。
(1) この論文は、ワクチン接種で高齢者の命を救えることを見事に証明した
(2) 高齢者は、自然感染よりもワクチン接種で免疫をつけるほうが安全
(3) ワクチン接種率が低い地域では、とくに60歳以上でコロナ死亡が多い
(4) 子供にワクチンを接種しても救われる命は少なかった
これらのコメントが正しいのか、検証してみましょう。まず「(1) ワクチンが多くの高齢者の命を救った」とするデータは、きわめて根拠が薄弱で、ほとんど無意味なものであることは前回の記事で述べたとおりです。
次に、「(2) 自然感染よりワクチン接種のほうが安全」とのコメントについては、議論のあるところかもしれません。ワクチン接種で免疫機能が乱れてしまい、むしろ感染しやすくなるというデータが多いことや、接種後の死亡が60歳以上の世代で圧倒的に多いという事実(資料3)をふまえると、やはり誤った主張と言えるでしょう。
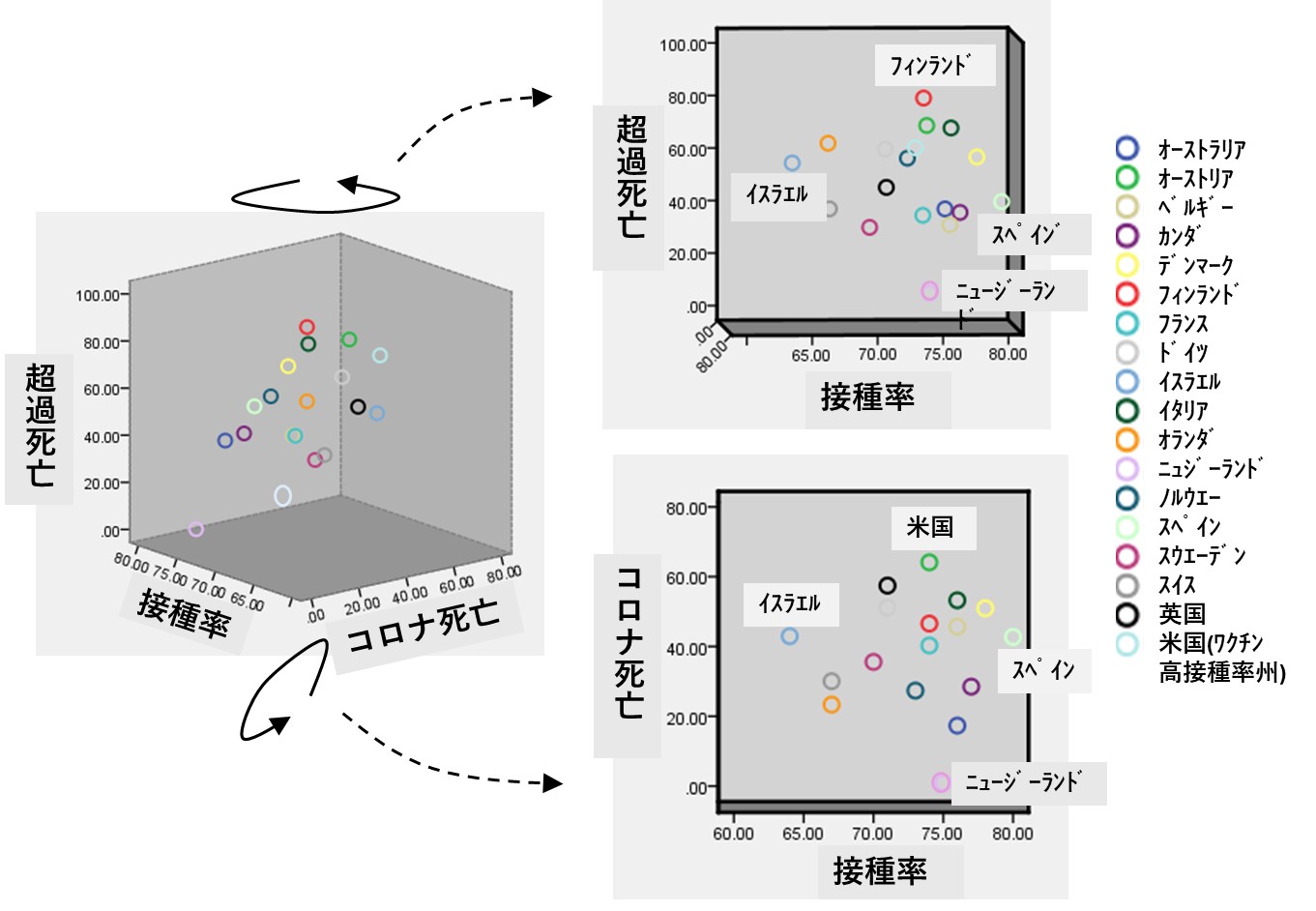
「(3) ワクチンを接種していない60歳以上でコロナ死亡が多い」とのコメントは、別の論文(文献4)を引用したものですが、年齢別の分析はなされていないことから、記述の誤りと思われます。ちなみに、その文献4は、世界20ヵ国と米国における「ワクチン接種率」、「コロナ感染による死亡率」、「超過死亡率(総死亡)」の各公表データを集計したものです。
以下の図は、同論文に掲載された数字を私が3次元グラフにしたものです(SPSSを使用)。超過死亡も、またコロナ死亡も、ワクチン接種率とはまったく無関係であることがわかります(相関係数が有意でない)。なお米国のデータは、接種率がとくに高かった10州の平均となっています。
なおグラフ中に日本のデータがありません。なぜか超過死亡の報告値が欠損しているためですが、「ワクチン接種率は最高の80%」、「コロナ死亡率は2番目に低値の10.4%」でした。
最後のコメント(4)は、子供の接種をどう考えるかという問題です。日本では生後6ヵ月以上が接種対象として推奨されていて(文献4)、立場は正反対です。
この問題を考える上で重要な研究があります(文献5)。昭和37年から昭和62年までの間、日本では小学校、中学校の子供たちを対象にインフルエンザワクチンの集団接種が行なわれていました。ご記憶の方も多いものと思います。
この点に着目した米国、日本などの研究者グループは、「ワクチン集団接種の時期」と「肺炎による死亡率の推移」を調べました。その結果、集団接種が始まる前までは、日本人の肺炎死亡率は開発途上国なみに高かったのですが、集団接種の期間中は激減し、中止後、再び増加に転じるという、際立った関係性のあることを見出したのです。
この事実が意味しているのは、常にウイルスは元気な子供たちの間で広まり、やがて同居の高齢者が感染し、肺炎など重篤な病気を発症している、ということにほかなりません。つまり感染症の大流行(パンデミック)を抑えることができるかどうかは、子供たちの感染をどう防ぐかにかかっていることになります。
ただし、「ではどうすればよいのか」という問いには、いまだ明確な答えがありません。いずれにしても、有名医学専門誌が偏見にもとづいた主張を依然として繰り返しているという、”憂うつ”が続いています。
【参考文献】
1) Loannidis JPA, et al., Global estimates of lives and life-year saved by COVID-19 vaccination during 2020-2024. JAMA Health Forum, Jul 25, 2025.
2) Gandhi M, COVID-19 vaccination saved lives and this matters in 2025. JAMA Health Forum, Jul 25, 2025.
3) 『新型コロナワクチン接種後死亡者の年齢別内訳』, 第7回 医薬品等行政評価・監視委員会, 参考資料5, 厚生労働省, Mar 18, 2022.
4) 『2024/25シーズンの小児への新型コロナワクチン接種に対する考え方』, 日本小児科学会, Oct 27, 2024.
5) Reichert TA, et al., The Japanese exprerience with vaccinating schoolchildren gainst influenza. N Engl J Med, Mar 22, 2001.
6) Bilinski A, et al., COVID-19 and excess all-cause mortality in the US and 20 comparison countries, June 2021-March 2022. JAMA, Jan 3, 2023.
(2025.9.1)
Q&A 「コロナワクチンが250万人の命を救った」って本当?
公表されているデータをもとに計算式を組み立て、表記のような結論を得たとする研究論文が発表されました(文献1)。本当でしょうか?
この研究は、米国スタンフォード大学とイタリアの研究者によって行われたもので、以下の数式によって「コロナワクチンによって救われた命の数が計算できる」と主張しています。
生存者数={人口}×{感染率}×{死亡率}×{ワクチン効果}
右辺にある4つの項目(変数)に代入する値は、公表されている以下のようなデータを用いたということです。
人 口: 世界総人口79億5449万人
感染率: 未接種者が対象で、オミクロン流行前で20%、同流行期が5%
致死率: 感染者の死亡率で、ワクチンがまだなかった頃のデータで1.8%
ワクチン効果: 死亡予防効果で、オミクロン流行前が75%、同流行期が50%
計算の結果、コロナワクチンを接種した人が、「もし接種していなかったら、全世界で250万人以上の死亡が増えていた」という結論になったというのです。
上記の数式と数値を使って、検算をしてみましょう。かりに人口が1万人だったとします。ワクチン未接種者の感染率がオミクロン流行前で0.2、そのうち死亡したヒトの割合が0.018ですから、死亡者数は以下のように計算できます。
10,000 × 0.2 × 0.018 = 36(人)
次に、もしワクチンの死亡予防効果が0.75だったとすれば、救われた命は、以下のような計算になります(ただし論文では、年齢階層別、オミクロン株流行前後などに分けて計算がなされ、あとで合算しているため、この式に世界総人口を単に当てはめても答えは合わない)。
36 × 0.75 = 27 (人)
計算方法はもっともらしいのですが、この論文には疑問点がいろいろあります。まず気になるのは、過去の文献から引用された統計データが正しいのかどうかです。特に重大な意味をもつのは「ワクチン効果」の値です。この値が間違っていれば、論文の主張はまったく成り立たないことになります。
この数値は2つの文献(2と3)から引用したと記されています。その文献2では、ワクチン効果を単純な割り算ではなく、年齢、性別、人種、居住地域などで補正した多変量解析で求めています。この点は評価できるのですが、その元になるデータが「後ろ向き調査」でしかありませんでした。
文献3のほうは、ランダム化比較試験のデータを集約したものでしたが、「ワクチンには総死亡を抑える効果がない」との結論を報じたものでした。
論文の骨格をなす数式についても、変数がきちんと定義されていなかったり、逆に数式で使われていない変数が、あとで無意味に説明されたりしているなど、理解が困難なのです。加えて、文章も稚拙で、ミスプリントもいくつかありました。一流とされる学術誌には文章校正のプロがいて最終点検を行っているため、ミスプリントはほとんどないのが普通です。
かつて私が米国の工学系専門誌の編集を担当していたころ、このような原稿に出会うことがしばしばありましたが、原稿を一旦、返却し、全面的な書き直しを求めるのが常でした。この論文を掲載したのは世界のトップクラスとされる専門誌ですが、果たして取り扱いが適切だったのか疑問です。
論文が掲載された同じ号には、これを絶賛する専門家のコメントが特別掲載されていました(文献4)。このコメントこそ偏見と誤解に満ち溢れたものでしたが、あたかも同専門誌の公式見解のごとく扱われているのです。内容については、次回、改めて紹介することにします。
【余談】
この論文と対極にあるのが、いま話題の 『映画:mRNAワクチンの内幕』です(文献5)。制作したのは米国のマッカロー財団で、当ホームページで紹介したこともあるロバート・W・マローン博士(2023/10/9付け記事)も関わっているようです。映画の内容は、すべて当ホームページで紹介ずみの情報ばかりですが、高精細のCGが見事です。日本語字幕つきですが、もし表示されなければ、動画の下方にあるボタンにカーソルを重ねていき、「字幕C」と表示されるものを1回クリックしてください。
【参考文献】
1) Loannidis JPA, et al., Global estimates of lives and life-year saved by COVID-19 vaccination during 2020-2024. JAMA Health Forum, Jul 25, 2025.
2) Lin DY, et al., Effectiveness of Covid-19 vaccines over a 9-mont period in North Carolina. N Engl J Med, Jan 12, 2022.
3) Korang SK, et al., Vaccines to prevent COVID-19: a living systematic review with Trial Sequential Analysis and network meta-analysis of randomized cinical trials. PLoS One, Jan 21, 2022.
4) Gandhi M, COVID-19 vaccination saved lives and this matters in 2025. JAMA Health Forum, Jul 25, 2025.
5) "Inside mRNA Vaccines: The Movie", https://www.youtube.com/watch?v=fDo5-fONeLg
(2025.8.25)
Q&A コロナワクチンでなぜ血圧が上がるのか?
「新型コロナのワクチン接種を受けたあと、血圧が上がったままで毎日が辛い」とのお便りが当ホームページあてに何通か届いています。この問題に改めてスポットをあててみることにしました。
新型コロナウイルスのスパイク蛋白は、ヒトの細胞表面にあるACE2という名の酵素に結合して細胞内に侵入します。この酵素は、血管の拡張や収縮に関わっていますから、血圧が変化するのは当然なのですが、問題はそれほど簡単ではなさそうです。
ワクチンの接種を受けたあと、血圧が上昇するパターンは2通りあります。一つは、注射の直後、数分以内に起こる反応です。たとえば白衣高血圧という言葉があるように、病院などで白衣を着た人に接するだけで血圧が上がってしまう人も少なくありません。ただし、よく知られたアナフィラキシーショックは、直後に血圧が下がって生じるものですから、まったく異なる現象です。
いずれにしても、これくらい短い時間で、ワクチンによって体内でスパイク蛋白が合成されることはありませんから、前述のACE2は関与していないことになります。
もう一つのパターンは、接種後、数時間から数日して血圧が上がってくるというものです。この問題を最初に報じたのは、スイスの研究者でした(文献1)。接種後30日以内に9人が重度の高血圧と判定され、7人が緊急救命室に運び込まれました。9人のうち8人は以前から高血圧症との診断を受け、治療により値は安定していたということです。すべての人で心拍数が増えていなかったことから、「白衣高血圧などは否定できるが具体的なメカニズムは不明だ」と、この研究者たちは報告しています。
ギリシャの研究者が行った検討では、ボランティアにあらかじめ自宅で血圧を測ってもらい140/90 mmHg以下だった50人を対象に、接種の24時間後に血圧を測っていました。その結果、全員が156/94 mmH以上に上昇していたということです(文献2)。
その後、同様の事例報告が相次ぐようになり、全体像が少し見えるようになってきました。それらの論文を集計し、最終的に35万人を超えるデータをまとめた、という研究があります。それによれば、3.2%の人に統計学的に有意な血圧上昇を認めたとのことです(文献3)。
接種後、1日以上経ってから生じる血圧上昇のメカニズムについては、以下のように考えられます。体内で血圧を上げる必要が生じた際に働く物質があり、アンジオテンシンIIと呼ばれています。上述したACE2は、アンジオテンシンIIをアンジオテンシンという物質に変換させるのが本来の役割で、変換後のアンジオテンシンは逆に血管を広げ血圧を下げるように働きます。
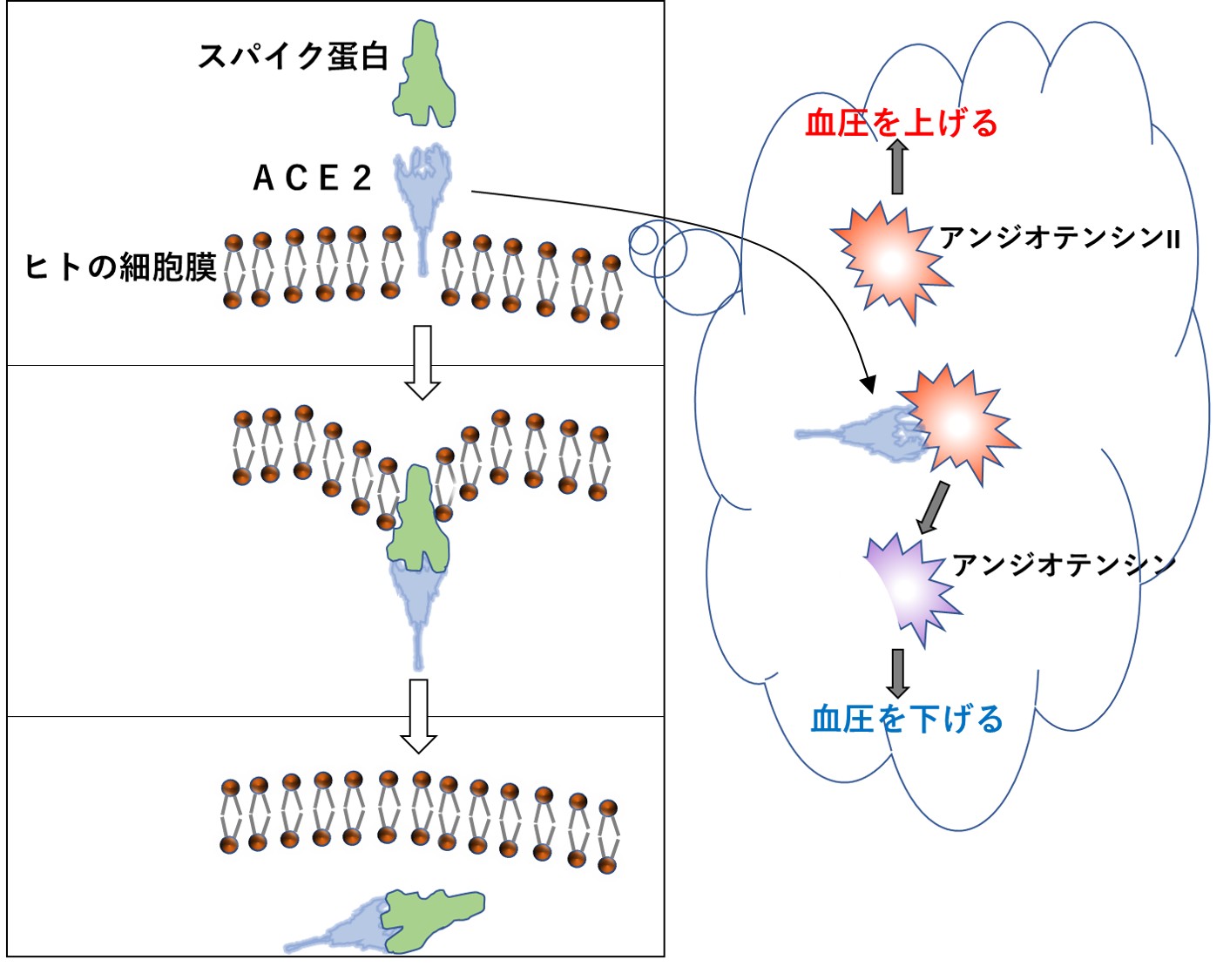
つまりコロナワクチンによって体内で過剰に作られたスパイク蛋白は、この大切なACE2に結合して細胞内に引き込んでしまうため、本来の働きが失われ、結果的に血圧は上がる一方となるのです。
ワクチンによって生じたスパイク蛋白は、接種後、長くとも半年後には体内から完全に消失します。したがって、それ以上続く高血圧はさらに別のメカニズムが働いていることになりますが、詳細は不明です。
コロナワクチンの接種を受けたあと、血圧の高い状態が続いている人は、高血圧の原因となる褐色細胞腫や腎動脈硬化症などの病気が、もともと隠れていた可能性もあり、これらの検査を受けておく必要があります。また喫煙や肥満なども血圧をあげる要因となります。
検査に異常がなく、かつ生活習慣の改善を行ってもなお血圧が下がらない場合でも、日々少しづつ体を動かしながら、生活を元の状態に戻していく努力をすることにより、血圧は徐々に改善していきます。これは多くの研究が示しているところです。
まだ多くの人が、コロナワクチンによる健康被害のため元の生活に戻れず、救いの手を求めています。
【参考文献】
1) Meylan S, et al., Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hyptertension, Jun, 2021.
2) Sanidas E, et al, Short-term blood pressure alterations after COVID-19 vaccination. J Hypertens, 40, e-Supplement 1, May, 2022.
3) Verdecchia P, et al., Cardiac complications of COVID-19 vaccination: now we know more. Eur Heart J, 24 (Supplement I), 2022.
(2025.8.18)
Q&A コロナワクチンのジレンマをAIが救うのか? 【第3回】
「人工知能で未来型のワクチンを作る」という研究発表があり、先々回からその方法について述べてきました。今回は、具体的な研究成果と意義について考えます。
mRNAタイプの従来型コロナワクチンは、スパイク蛋白全体を細胞内で作るよう設計されていたため、深刻な副作用から逃れることができませんでした。新型コロナの発病予防に必要なのは、実はスパイク蛋白全体でなく、その先端部にあって、ヒトの細胞表面の受容体と結合する、きわめて小さな部位(受容体結合部)です。
この研究は、受容体結合部に着目し、そこだけに結合する極小蛋白をAIで設計することを試みたもので、主な結論は以下の4点でした(文献1,2)。
・有力候補を、数兆に及ぶ組み合わせから92種類に絞ることができた
・遺伝子組換えで作った極小蛋白は、試験管内で実際に受容体結合部と密着した
・うち2個は最初に流行した新型コロナウイルスのスパイク蛋白に強く結合した
・また、ほかのいくつかは、最新の流行株のスパイク蛋白にも結合した
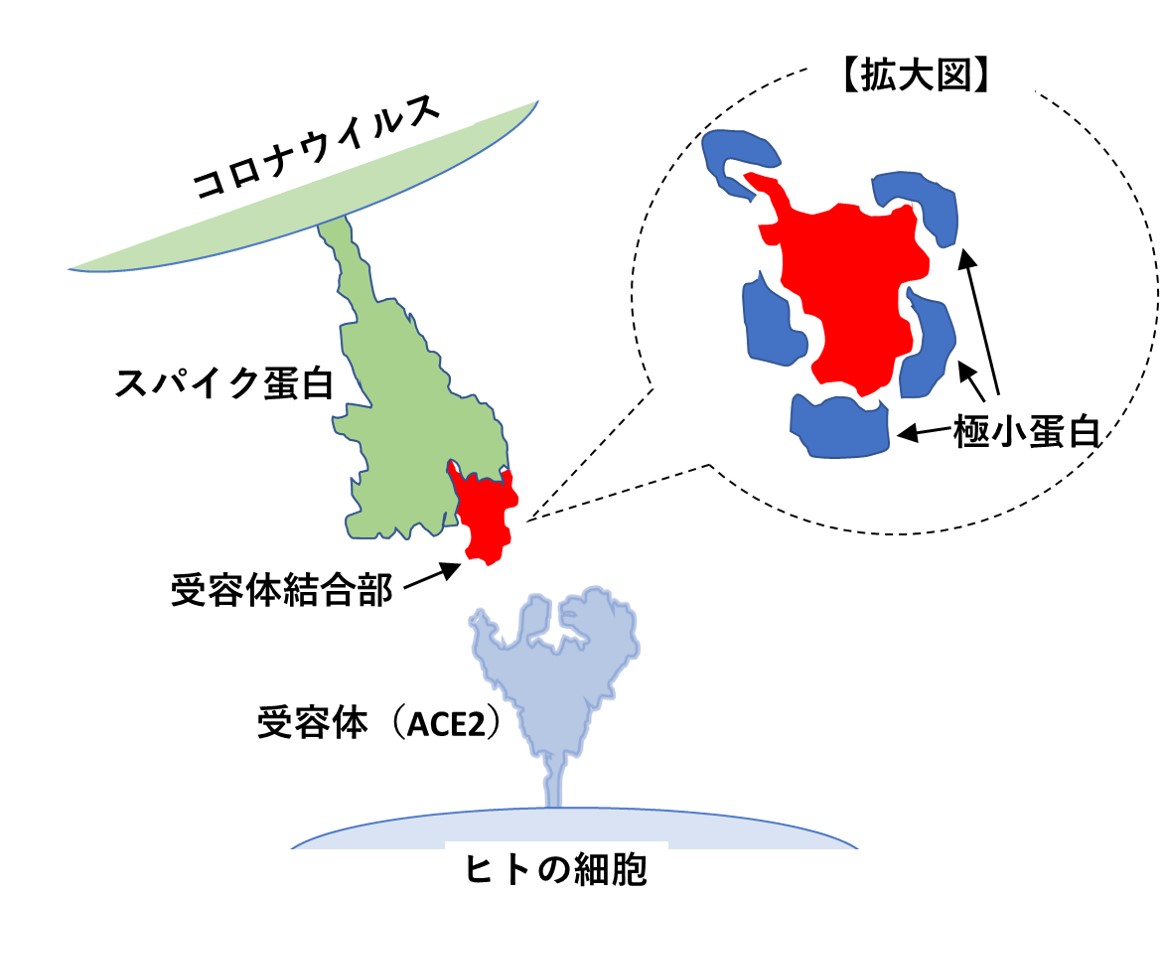
これら極小蛋白をどのように臨床応用するかについては、論文中に何も記載がありませんが、考えられる応用は2つです。ひとつは、これらの「極小蛋白」を遺伝子組換えで大量に作り、抗体療法の注射薬として利用する方法です(文献3)。
新型コロナが流行し始めたころ、「感染者が回復したあとの血清に含まれる抗体は、予防や治療に役立つはず」との発想から、実際に血清を用いた臨床試験がいろいろ行われました。しかし、いずれも期待した効果が得られず、実用化することはありませんでした。
同じころ抗体カクテルという言葉も話題になりました。しかし製造工程が複雑で、ウイルスの急速な変異に合わせて改造していくことができなかったため、いまは使われていません。いずれにしろ、抗体そのものを注射するという方法はアイデア倒れだったのです。
もうひとつ考えられる応用は、(極小蛋白ではなく)受容体結合部のほうの対応部位をコードするmRNAを作り、ワクチンとして注射するという方法です。従来のコロナワクチンと異なるのは、ヒトの体内で再合成される物質が、スパイク蛋白の全体ではなく、受容体結合部の一部分に限定されることです。
ただし、このアイデアにも問題があります。ヒトの免疫システムは、小さすぎる異物(抗原)に反応できないため、抗体が産生されない可能性があります。たとえ何らかの工夫で、抗体ができるようになったとしても、スパイク蛋白がもたらす深刻な副作用が回避されるかどうかは、まったく不明です。すべての臨床試験を、犠牲を払ってでも最初からやり直さなければならないことになりそうです。
ワクチンが運命的に背負っている諸問題を解決するのは簡単でありません。
【参考文献】
1) Swanson K, et al., The virtual lab of AI agents designs new SARS-CoV-2 nanobodies. Nature, Jul 29, 2025.
2) Swanson K, et al., The virtual lab: AI agents design new SARS-CoV-2 nanobodies with experimental vlidation. bioRxiv, Nov 12, 2024.
3) Lapid N, Virtual scientists come up with possible vaccine improvement. Reuters Health Rounds, Jul 30, 2025.
(2025.8.11)
Q&A コロナワクチンのジレンマをAIが救うのか? 【第2回】
次世代のコロナワクチンが人工知能(AI)で作れそうだ、という話題を先回、紹介しました。今回はその続きで、「なぜワクチンにAIなのか」を考えます。
AI研究の歴史は以外に古く、1959年にはすでに最初のアイデアが提唱されています。以来、何回かのAIブームを経て現在に至っているのですが、技術の進歩が著しいことに加え、スマホで気楽にアクセスできるアプリも登場し、いっそう熱気を帯びているのは、ご存じのとおりです。
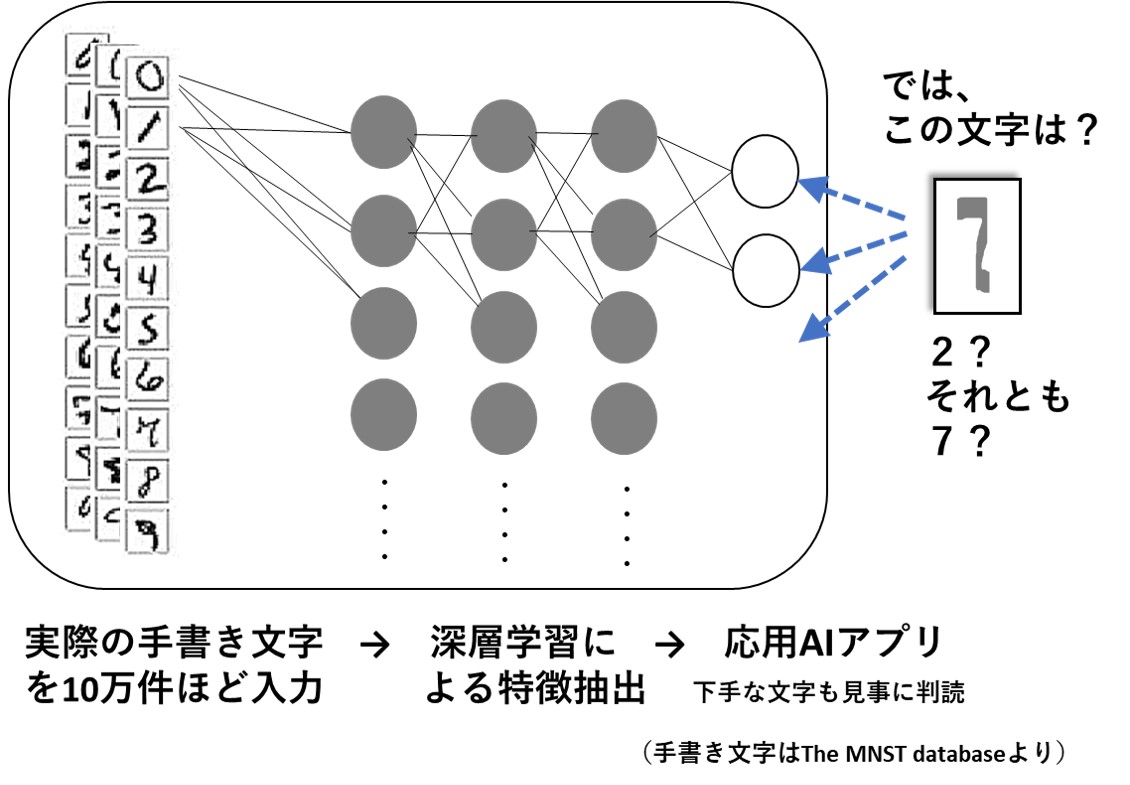
最先端のAIを支えているのは、2つの技術です。ひとつは、現実に存在する文章や画像などの膨大なデータを集め、その特徴を数学的に抽出しておき、未知のデータに遭遇した際にコンピュータで判別できるようにするというものです(文献1)。
そのためには、人間が気づかないような、わずかな違いや特徴を抽出する必要があり、膨大な数のデータに対して無数の計算が繰り返されます。その様子から、深層学習などと呼ばれています。わかりやすい応用例は、手描きされた手紙の郵便番号を自動的に判読するAIシステムでしょう。次の図は、そのイメージです。
もうひとつの革新的技術は、文章の中の一つ一つの単語について、次にくる言葉を予測する方法です(文献2)。まず、無数の文章を事前に学習し、単語と単語のつながりの強さを数値化しておきます。AIは、文章の意味を理解しているわけでなく、個々の単語(あるいは文字)に注目し、それぞれの出現頻度や関係性、あるいは並べ方を学習し、その結果にもとづいて予測を行うという戦略なのです。
この2つの技術を利用して成功を収めたAIアプリは多数ありますが、よく知られたチャットGPTはその代表です。2024年のノーベル化学賞授与の対象となった,遺伝コードからたんぱく質の立体構造を予測するAIアプリもそうです。
新型コロナ感染症で問題となるスパイク蛋白やその受容体、あるいは免疫反応にかかわる抗原や抗体など、どれもたんぱく質であり、形の似たものどうしがくっついたり離れたりすることで「生体反応」が進んでいきます。したがって、その形状を知ることは、非常に重要なのです。
さて、先回から紹介している研究も、これらのAI技術を組み合わせたもので、論文のタイトルは『AIエージェントのバーチャルラボがデザインするコロナウイルスの超微小抗体』です(文献3,4)。「バーチャルラボ」は仮想実験室のことで、「エージェント」は代理人という意味ですが、秘密諜報員を表す言葉としても使われます。要するに、カッコつけるために流行りの言葉を並べたわけです。
前述したいくつかのAIアプリを操作するのは、別に作成した5つのAIアプリ(エージェント)で、これに人間が一人加わって1チームをなしています。
人間が発した最初の指示をもとに、主任エージェントは、各AIエージェントに指示を与え、試行を何回か繰り返したのち、エビデンス認定エージェントが総合判定をするようになっています。その後、チームミーティングが行われ、最終結論にいたる、ということのようです。以下は、そのイメージですが、ほとんど漫画の世界です。
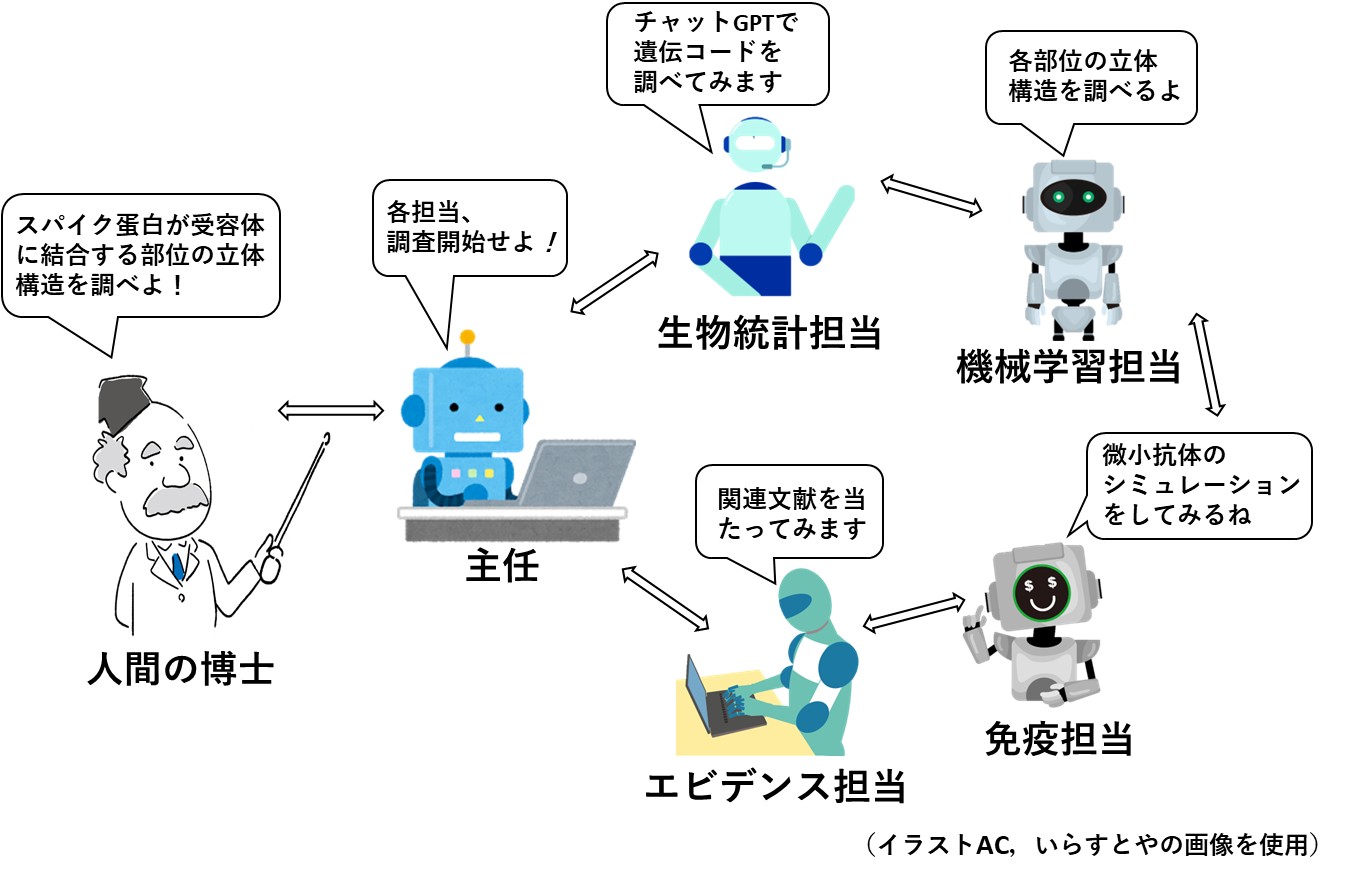
研究の信頼性は非常に高いと判断されますが、得られた結果が複雑な人間の体内で思い通りに機能し、コロナワクチンの問題点(ジレンマ)を解決してくれるかどうかは、別問題です。最終的にどのような発見がなされ、ワクチンとして有望なのかどうかについては、(予定を変更して)次回、まとめることにします。
【参考文献】
1) 岡田正彦,『医療AIの夜明け―AIドクターが医者を超える日』, オーム社, 2019.
2) Vaswani A, et al., Attention is all you need. 31st Conference of Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 2017.
3) Swanson K, et al., The virtual lab of AI agents designs new SARS-CoV-2 nanobodies. Nature, Jul 29, 2025.
4) Swanson K, et al., The virtual lab: AI agents design new SARS-CoV-2 nanobodies with experimental vlidation. bioRxiv, Nov 12, 2024.
(2025.8.4)
Q&A コロナワクチンのジレンマをAIが救うのか?
有名な科学専門誌Natureに『AI実験室が新型コロナウイルスの新しい抗原を発見』と題する論文が掲載され、その冒頭、編集委員会からのコメントとして、「この重要な論文を一刻も早く読者にお届けするため、未審査のまま掲載しました。たとえ間違いが含まれていたとしても、正式な審査が終了するまでは、読者の責任でお願いします」と、記されていました(文献1)。前代未聞のコメントです。
いったい、どんな内容の論文だったのでしょうか? 実はNature誌に掲載される8ヵ月ほど前、同じ内容の論文が「審査なしで学術論文を掲載するサイト bioRxiv」上で発表されていました(文献2)。この2つの論文を合わせ、今回から2回にわけて概要を報告します。
従来のコロナワクチンには、三つの重大な問題がありました。第1は、ワクチンの主成分である遺伝コード(mRNA)に、危険なたんぱく質を作る情報が(偶然に)含まれていたことです。代表は、クロイツフェルト・ヤコブ病という致死的な病気の原因となるプリオンという物質です。第2は、mRNAを包む脂質微粒子に、急性毒性をもたらす物質(pH応答性脂質)が使われていたことです。
そして第3が、(ワクチンによって細胞内で合成される)スパイク蛋白、そのものが危険だったことです。このスパイク蛋白は、ワクチンとして抗体産生をうながすだけでなく、健康被害をもたらす重大なリスクをかかえていました。ウイルスが細胞内に侵入する際、最初に会合する部位は受容体と呼ばれますが、新型コロナの場合、それがある種の酵素(ACE2)で、そこにスパイク蛋白が接着することが知られています。
この酵素は本来、血管細胞の表面にあって血圧を適切に保つ調節を行っているものと考えられています。ところが、なぜか全身のさまざまな細胞表面にも存在し、スパイク蛋白が結合することによって、結果的に自己免疫病、心筋炎、腎炎、悪性腫瘍などを引き起こすきっかけとなっていることがわかってきました。
スパイク蛋白には、もう一つ問題があります。ヒトの細胞表面には、免疫システムから自分自身が攻撃を受けないよう「自分であることの目印」があります。そのひとつがシアル酸と呼ばれる物質ですが、スパイク蛋白は、これを切断する酵素としても働くため、自己免疫反応が起こってしまい、さまざまなざまな異常、たとえば止血作用のある血小板が破壊されて、脳出血が起こったりすることになります。
では、なぜ一流の世界的製薬企業が、これほどまでスパイク蛋白にこだわってワクチンを開発し続けてきたのでしょうか?
理由はあきらかです。新型コロナウイルスがヒトの細胞内に侵入するのをブロックするには、スパイク蛋白(抗原)が受容体とくっつかないようする中和抗体を免疫反応で作らせるというアイデアしか持っていなかったからです。元来、ワクチンとは、そのような単純な発想にもとづくものです。
もうひとつ、なぜ誰もスパイク蛋白の危険性に気がつかなかったのかという疑問もあります。この疑問に対する答えもあきらかで、そのような研究はこれまでまったくなされておらず、世界中の人々に実際に接種してみて初めてわかったことだったからです。
さて、冒頭で紹介したNature誌掲載の論文は、人工知能を使って、この問題に切り込んだという内容になっています。結論を先に紹介すれば、スパイク蛋白をコンピュータ上で分解し、数兆におよぶ遺伝コードの組み合わせの中から、ごく微細な立体構造を92ヵ所について再現することに成功。次世代ワクチンの候補になりそうだ、というものです。
いま大流行の人工知能ですが、その仕組みを多少なりとも理解しておかないと、誤った話を押しつけられることになってしまいます(文献3)。次回は、この論文で使われた人工知能がどのようなものだったのか(文献4)、スパイク蛋白のジレンマを解決することはできるのか、そして次世代の医療に役立つのかなど、わかりやすい解説を試みる予定です。
【参考文献】
1) Swanson K, et al., The virtual lab of AI agents designs new SARS-CoV-2 nanobodies. Nature, Jul 29, 2025.
2) Swanson K, et al., The virtual lab: AI agents design new SARS-CoV-2 nanobodies with experimental vlidation. bioRxiv, Nov 12, 2024.
3) 岡田正彦,『医療AIの夜明け―AIドクターが医者を超える日』, オーム社, 2019.
4) Lapid N, Virtual scientists come up with possible vaccine improvement. Reuters Health Rounds, Jul 30, 2025.
(2025.7.28)
Q&A X(旧twitter)に投稿された58秒の動画で大騒動?
米国保健社会福祉省(HHS)のケネディ長官と、アメリカ食品医薬品局(FDA)のマカーリ長官など3人が2025年5月27日、ワクチンに関する短い動画をX.comに投稿。今回は、それが元で大騒ぎになっているという話題です(文献1)。
この動画サイトには、すでに7千件を超えるコメントが投稿されていますが、ある医学専門誌にも、編集委員会の専属ライターによる論文が掲載されました(文献2)。
動画の主旨は、「FDAは、健康な妊婦にコロナワクチンの接種を勧めない決定をした」というものでした。それに対する文献2の論文は、動画の中にCDCの関係者がいなかったこと、およびFDAが大方針転換をしたにもかかわらず、科学的根拠がまったく示されていなかったことを厳しく批判する内容となっています。
論文の後半は、専門家と称する人たち、とくにFDAやCDCの専門委員を解雇された人たちによるコメントで埋め尽くされていました。以下は、その一部です。
「自分は、数えきれないほど多くの妊婦にコロナワクチンを接種してきたが、問題が生じたことは一度もなかった」、「新型コロナに感染することは、本人だけでなく、生まれてくる赤ちゃんや、さらには家族全員に破滅的な被害をもたらす」、「われわれ学術団体は、FDAやCDCと縁を切り、ワクチン接種の指針を科学的根拠にもとづいて、独自に作成するつもりだ」などです。
この論文に対しては、専門誌の編集部にも多数のコメントが寄せられ、そのうち7通が掲載されています。その1通は、自分の子供がコロナワクチンで健康被害を受けたという母親からの投稿で、「この記事が本当かどうかは判断できないけれど、誠実に書いてないことだけは確か」というものでした。
別の一般女性からは、「妊娠中は、いかなる薬も使わないことが大原則。mRNAワクチンも例外じゃない」。また、一般男性からの投稿では、「この論文はコロナワクチンを宣伝するためのものだ。専門家と称する人たちは、副作用など正しい情報を隠ぺいしたまま、大衆の肉体をメーカーに売ったようなもの」と厳しい意見が記されています。
このような意見は、これまでSNS上で見聞することはあっても、医学専門誌に掲載されるのは前代未聞です。
このマカーリ長官は、実は動画を投稿するわずか1週間前、医学専門誌に論文を投稿し「コロナワクチンは抗体価を高める効果が立証されているので、妊娠などのリスク要因を有する(生後6ヵ月以上)のすべての人に、接種を推奨することになるだろう」と書いていました(文献3)。つまり主張が1週間で180度、変わったのです。
およそ2ヵ月後、マカーリFDA長官らは、さらに別の医学専門誌に、『mRNAに関するFDAの方針変更について』と題する論文を投稿しました(文献4)。コロナワクチンの副作用として広く知られている心筋症の研究データをまとめたものですが、いささか旧聞に属し、あきらかに批判をかわすためだけの文章でした。
通常、学術論文は、原稿を投稿したあと厳しい審査を受け、多くは却下され、一部の原稿だけが修正を繰り返し求められたのち、1年後くらいに掲載となります。しかし、この論文は、現政権によって選ばれた人からの投稿でしたから、無審査で即日受理されたようです。「政権に逆らうと報復を受ける」のは、学術専門誌でさえも例外でなかったようです。
この話題を今回、取り上げたのは、コロナワクチン問題に白黒決着をつけるためではなく、アメリカ社会の混乱と、醜聞と、そして凋落ぶりを紹介するためでした。アメリカに百パーセント依存してきたわが国の医学・医療ですが、立ち止まって考えるべき時にきたようです。
【参考文献】
1) https://x.com/SecKennedy/status/1927368440811008138
2) Rubin R, The CDC no longer recommneds COVID-19 shots during pregnacy - now what? JAMA Netw, Jul 11, 2025.
3) Prasad V. and Makary MA, An evidence-based approach to Covid-19 vaccination. N Engl J Med, May 20, 2025.
4) Prasad V, and Makary MA, US FDA safety labeling change for mRNA COVID-19 vaccines. JAMA, Jul 14, 2025.
(2025.7.21)
Q&A モデルナ社の謎のコロナワクチンが米国で承認?
2025年6月5日、モデルナ社は、「新しいコロナウイルスのワクチンmNEXSPIKEの製造販売承認をFDAより得た」とのメッセージをホームページ上で公開しました(文献1)。今回は、このニュースの背景を探ってみます。
承認の内容は、65歳以上のすべて人と、基礎疾患を有する12~65歳に限るという条件つきでした。コロナワクチンについては、今さら感もあり、これを報じたメディアも多くありませんでした。しかも、どの報道もメーカーの広報をそのままに、「これまでのmRNAワクチンと比べて効果は劣らない」 、「深刻な副作用は認められなかった」などの記述に終始しています(文献2)。
このニュースについては不審な点がいくつかあります。まず、このワクチンが、どのようなメカニズムで働くのかについて、メーカーの広報には説明がなく、各メディアもまったく触れていないことです。
ある学術誌に、唯一、次のような記事が掲載されていました(文献3)。「この度、FDAが承認したmNexspikeは、まったく新しいメカニズムにもとづく自己増殖型RNAワクチンであり、それにも拘わらず、単に従来のmRNAワクチンの改良品としての審査しかなされていない。安全性に関する徹底した検討が必要だ」
この記事が正しければ、すでに当ホームページ(2025年2月24日と2024年9月30日付け記事)でも取り上げたレプリコン・ワクチン、そのものということになります。当時、日本政府が、世界に先駆けて、このタイプのコロナワクチンを承認したことから、騒動となったのは記憶に新しいところです。そのニュースは、当然、米国にも届いていたはずですから、FDAとメーカーの不審な言動もうなずけます。以下は、以前の記事で使用した図の再掲です。
このワクチンの審査には、最近のアメリカ社会の状況が色濃く影を落としています。すでに、アメリカ食品医薬品局(FDA)とアメリカ疾病予防管理センター(CDC)の2つの組織が保健行政の実務を担う国家機関でありながら、縄張り争いがあり、かつ医療業界に対して利益相反が生じていることは、当ホームページでも繰り返し述べてきたところです。
この関係を、さらにややこしいものにしているのが、アメリカの現政権です(文献4)。名門ジョンズ・ホプキンズ大医学部の教授からFDAの長官に抜擢されたマーチン・マカーリー氏は、部下に任命したヴィナイ・プラサド氏とともに、従来のFDAの方針を次々に覆しています。
二人が打ち立てた新しい方針のひとつは、「コロナワクチンの接種は65歳以上の世代に限るべきであり、それ以下の若い世代については、医師が重大な基礎疾患があると診断した場合のみ」というものでした。理由は、コロナ・ワクチンには、まだ十分に解明されていない副作用があるから、ということのようです。
モデルナ社の新しいコロナワクチンは、エビデンスの十分な精査が行われないまま、この二人の人物が掲げた方針に沿って認可がなされたのではないかと想像されます。このコロナワクチンが日本に輸出される日も近いと思われますが、どんな宣伝文句で登場してくるのか、注視する必要がありそうです。
なお、このワクチンについては十分な情報がなく、当記事にも不正確な点があるかもしれません。何か情報をお持ちの方は、ご一報をお願いします。
【備考】 新しく刊行された学術誌(文献3)について
「間違った医療を正す」という方針で刊行されたこの専門誌は、学術誌としての存在をまだ広く認知されておらず、「偽科学をまき散らす邪悪な媒体」との評価もあるようです。本文で紹介した記事の信ぴょう性についても、若干の疑問があることを付記しておかなければなりません。
【参考文献】
1) Moderna receives U.S. FDA approval for COVID-19 vaccine mNEXSPIKE. Moderna, May 31, 2025.
2) Moderna's new COVID-19 vaccine mNexspike approved by FDA, but there's a limit on who can use it. CBS News, May 31, 2025.
3) More on Moderna's mNexspike approval: a strategic inflection point for vaccine policy? Science, Public Health Policy, and the Law, 2025.
4) Jewett C, Top F.D.A .offical overrode scientists on Covid shots. New York Times, July 2, 2025.
(2025.7.14)
Q&A ものは言いよう! データに騙されない知恵とは?
「18歳以上のすべての人にコロナワクチンは有効で、これからも接種を続けるべき」と結論した論文が、また発表されました(文献1)。米国内の多くの医療機関から10万人を越すデータを集め、ワクチンの有効性を分析したという研究内容ですが、本当でしょうか?
今さらながらの研究論文が専門誌に掲載された理由は、オミクロン株の流行が主流となったころ(2023~2024年)にスポットを当てたことと、ワクチン接種の有無により重症化した人の割合がどう違うかを分析した点にあったようです。
以下のグラフは、その結果をまとめたものです(論文に掲載されている図を私がイラストで再現)。対象は18~64歳です。縦軸の値が0.0以上であれば、ワクチン接種は重症化予防に有効、マイナスであれば逆効果であることを意味します。
結果は当然、一人一人で異なります。各棒グラフの高さは有効率ですが、重ねて表示した縦の赤い線分の幅は、統計計算で得られるもので「結果の確からしさ(誤差範囲)」を表しています。
たとえば点線で囲んだ棒グラフは、120日(約4ヵ月)以降の効果ですが、赤線が0.0より下方にも伸びていて、「効果ありとも、なしとも言えない状態」です。したがって統計学では、「有意な効果があるとは言えない」との結論になります。
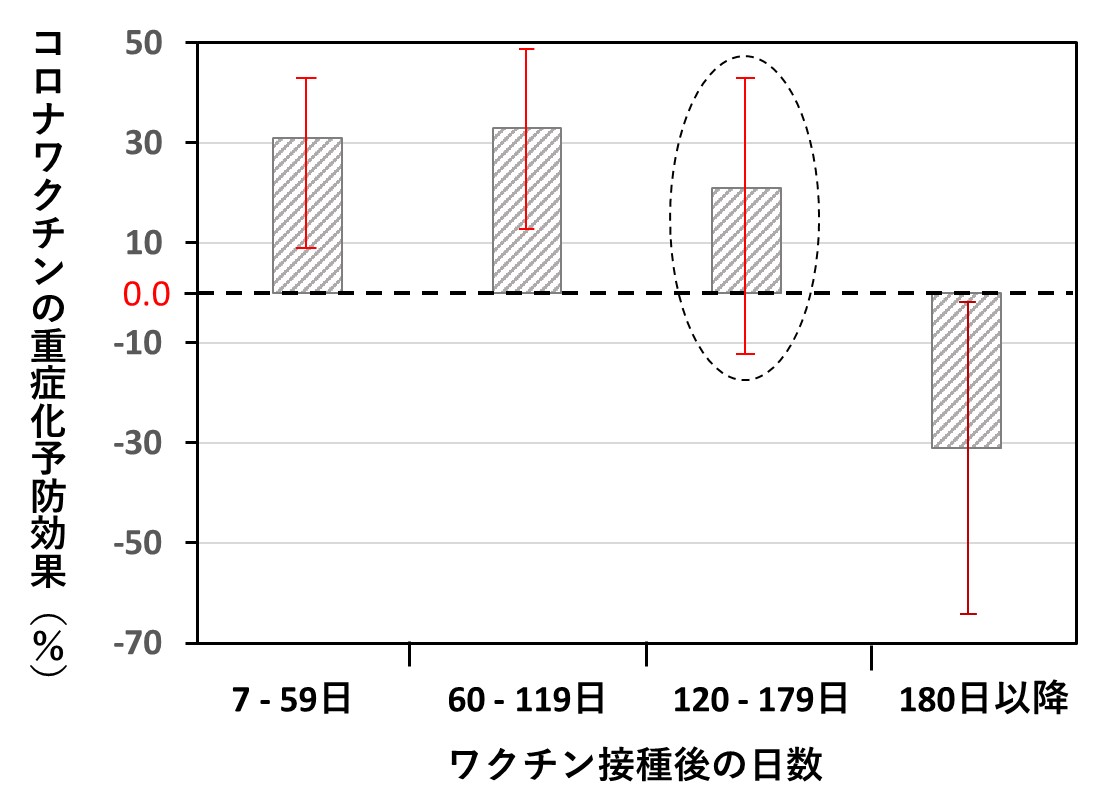
このデータのポイントをまとめると、以下のようになります。
・接種後120日(約4ヵ月)の間に限り、有効率が30%程度となる
・接種後180日(約半年)過ぎると効果は消失し、むしろ重症化率が30%高まる
ちなみにファイザー社が2020年に発表した論文では、有効率が95%となっていました。効果がほとんど認められなかったことに加え、この研究には重大な欠陥を2つ指摘することができます。
まず、テスト・ネガティブという一種の後ろ向き調査でなされていたことです。この方法については当ホームページの2023年5月9日付け記事で解説しましたが、PCR検査を受けた人たちだけを対象に、結果が陽性だった人たちと陰性だった人たちを比べるというものです。
この方法を評価する専門家は意外に多く、「検査を受けにきた」という行動が2群の間で共通しているので、比べることに問題はないと主張しています。しかし、たとえば陰性だった人たちは、日ごろからマスクを着用し、陽性だった人たちはそうでなかったかもしれません。年齢、性別、生活習慣など結果に影響を与えるような背景因子をそろえたわけではありませんから、そもそも比べてはならないものなのです。
もう一つの欠陥は、ワクチンの副作用によって重大な健康障害が起こっている点にまったく触れていないことです。たとえば副作用で死亡した人は、PCR検査を受けに来るわけもありませんから、ワクチンにとって不都合な事実が最初から無視されているのです。
この論文が結論とした「ワクチンは有効で、これからも接種を続けるべき」との主張は、著しく妥当性を欠くものと言えます。このような(結論ありきの)研究論文や論評がこれからも世の中に出てくるものと思われますが、データの真偽を見分ける眼力は失わないようにしたいものです。
【参考文献】
1) Link-Gelles R, et al., Estimated 2023-2024 COVID-19 vaccine effectiveness in adults. JAMA Netw Open, Jun 25, 2025.
(2025.7.7)
Q&A 新型コロナとインフルエンザ、どっちが危険?
当ホームページあてに、次のようなお便りがありました。ある地方紙に、『コロナの死亡率はいまだ他の感染症よりも高い。重症化リスクの高い高齢者らにはワクチン接種を推奨している、との医師会長談話』という記事が掲載され、気になりました、という内容でした。
このテーマについては当ホームページでも、これまで以下のようなコメントを掲載してきたところです。まず、新型コロナの流行が始まるとすぐ「2類感染症」に指定され、検査で陽性になった人は指定された医療機関に入院しなければならず、また医師はすべての感染者を保健所に届けなければなりませんでした。
そのため、たとえば交通事故で死亡しても、入院時に新型コロナ検査が陽性であれば、コロナ死としてカウントされてしまったとの批判もありました。行政は、このような事例の存在を否定していましたが、私自身の経験で言えば、高齢で老衰死が近いと診断した人がPCRで陽性となり入院。しばらくして入院先から「コロナで死亡」との連絡が入ったというケースが何例かありました。
逆に、実際に感染していても、検査が行われない人も少なくなかったのではないかと考えられます。いずれにしても、行政から発表されるコロナ死の統計には、大いなる疑いがあったのです。
さて、これらの混乱を一掃するかもしれないという研究発表がありました(文献1,2)。弱毒化したオミクロン株の流行期にスポットをあて、入院した患者に特化し、かつ入院後30日目まで追跡を続けて死亡の有無を確認したというものです。
交通事故に遭い、たまたまPCRが陽性だったような患者も、入院中にさまざまな検査が行われ、あるいは不幸にして亡くなったあとは病理解剖も行われたはずです。したがって新型コロナの感染と死亡との因果関係も、より確かだったでしょう。
この研究には、さらに特筆すべき点が2つありました。ひとつはインフルエンザによる致死率と比較をしていることです。致死率は、医療機関側がその感染症に慣れていたかどうか、あるいは患者が殺到し医療崩壊状態ではなかったかなど、社会的な要因も絡んできます。
しかし、同じ時期に入院した2つの種類の感染症に対する社会的背景は、たとえ致死率に影響を与えていたとしても、両者に共通する部分が多いため、「比べる(比をとる)」という操作を行うことで背景要因が互いにキャンセルされることになります。
もうひとつは、患者個人の背景因子を徹底的に調べ、その影響を新型コロナとインフルエンザの両データから統計計算によって取り除くという処理が行われていることです。たとえば年齢、性別をはじめ、居住地、肥満度、喫煙歴、既往歴、腎機能、血圧など調査は28項目にも及びました。
統計計算でもっと重要なのが、このような背景因子の影響を両群から平等に消し去り、影響をなくすという操作で、多変量調整と呼ばれます。
最終的にわかったのは、インフルエンザの致死率が3.74%で、新型コロナのそれは5.98%だったということです。新型コロナのほうの致死率が1.6倍ほど高いという結果でした。65歳以上とそれ未満の人たちに分けても、値はそれぞれ同じでした。
しかし当時、日本では、インフルエンザに関する医療機関や介護施設あての行政文章に、「1週間内に死亡者または重篤者が2名以上いた場合、保健所に報告すること」と記されていました。つまり、ここまで述べてきた諸問題に加え、感染症の種類によって届け出の基準までもが異なっていたのです。
結局、新型コロナの真の致死率は、いまのところ不明です。少なくとも、「コロナによる死亡者数はいまだインフルエンザのそれより遥かに多い。だから、これからもワクチン接種を!」との発言は、科学的根拠にもとづいていないことになります。
(本記事は、当ホームページあてにお知らせいただいた情報をヒントに構成したものです)
【参考文献】
1) Xie Y, et al., Risk of death in patients hospitalized for COVID-19 vs seasonal influenza in fall-winter 2022-2023. JAMA, May 16, 2023.
2) Sidharthan C, A comparison of death rates associated with COVID-19 versus influenza during fall-winter 2022-23. News Medical Life Sciences, Apr 10, 2023.
(2025.6.30)
Q&A 脳の病巣に残っていたコロナワクチンの痕跡?
mRNAタイプのコロナワクチンによる最初の被害者は、接種後の脳出血で死亡した米国の男性医師でした。そのニュースが米国の有力紙に掲載されたのは、ワクチン接種が医療関係者を優先する形で始まったばかりの頃です(文献1)。しかし、この出来事が日本国内で報じられることは、いっさいありませんでした。
脳出血を起こした19例(うち16例にワクチン接種歴あり)について、スパイク蛋白の有無を調べたという事例報告が日本からなされました(文献2)。脳出血の手術中に採取された組織の一部が保存されていた事例、あるいは死亡後に病理解剖が行われた事例です。今回は、この貴重な研究発表について概要をまとめます。
ワクチンとの因果関係を考える上で、まず重要なのは、新型コロナ感染症による影響(後遺症)と厳密に区別しなければならないことです。つまりワクチンには含まれず、感染した人にのみ認められるたんぱく質(抗原)が体内にあったかどうかです。
次の図は、新型コロナウイルスの構造を表わしたものです(以前の当ホームページで紹介)。図で示すS蛋白(スパイク蛋白)のみが検出されればワクチンのせいということになり、それ以外のたとえばN蛋白などがいっしょに検出された場合、感染もしていた証拠になるため、ワクチンだけの影響かどうかがわからなくなってしまいます。
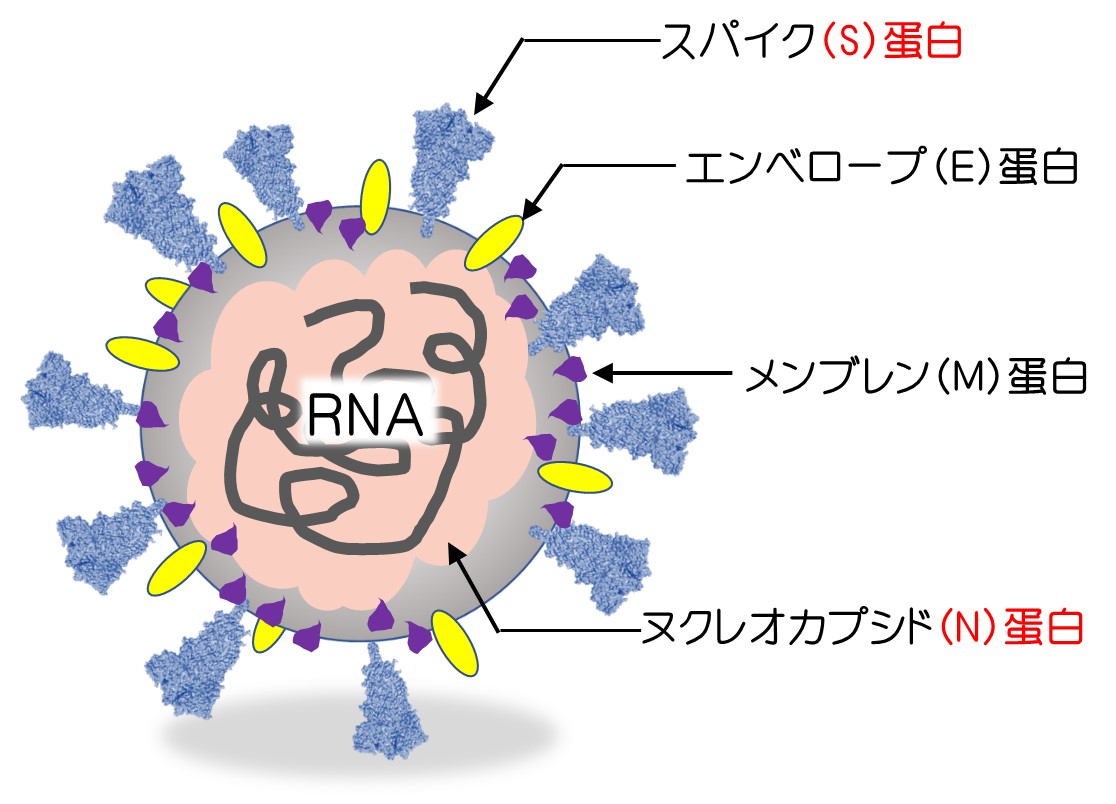
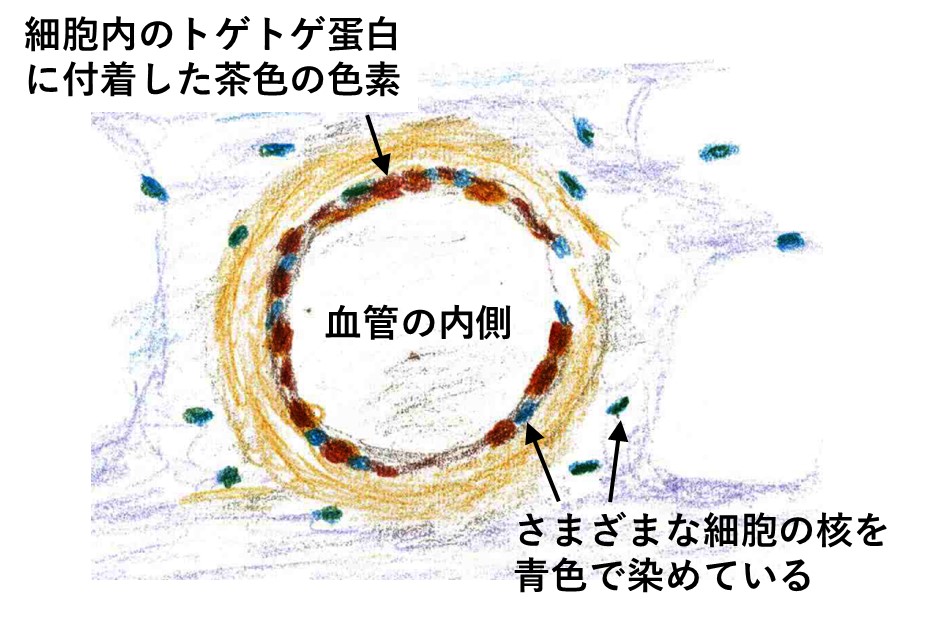
研究の結果は次のようなものでした。脳出血を起こした周囲の血管壁にスパイク蛋白を認め、かつN蛋白が見つからなかったかった事例が9例あり、それ以外はワクチン接種を受け脳出血を認めていたにもかかわらず、スパイク蛋白が検出されませんでした。また一部の事例では、mRNAそのものを特殊な方法で染色し映像化するという方法で検証が行われ、ワクチンの痕跡もいっしょに確認されたとのことです。
ワクチンと脳出血との間の因果関係を証明するための、もうひとつの要件は、血管や内臓の組織に、炎症反応や免疫反応が起こっていることです。もし反応が何も起こっていなければ、体に悪影響はなかったことになるからです。
この研究では、その点についても詳しい分析がなされ、スパイク蛋白の周辺に数種類の免疫細胞が集まっていることが顕微鏡画像で確認されています。論文には実際の顕微鏡写真が提示されていますが、以下の図はそのイメージを私が手書きしたものです(当ホームページの以前の記事で掲載したもの)。
ワクチンのせいで脳出血を起こした人が確かにいることが、これで証明されたと考えてよさそうです。問題点として残るのは、たとえスパイク蛋白の存在を認めたとしても、潜在的に感染していたかもしれないことを完全には否定できない点です。この問題については、さらなる検討が必要かもしれません。
コロナワクチンの接種後に脳出血が起こりうる事実は広く認められてきたところですが、従来の研究報告は、どれも「自然の発生率より低い」、あるいは「感染した人で起こる頻度のほうが高い」と結論したものばかりでした。(文献3)。
しかし、かりに自然の発生率よりも少なかったとしても、原因不明の脳出血例が多い中で、因果関係があきらかな発症を認めた事実は重く受けとめなければなりません。そして、かりに感染が原因で脳出血を発症する頻度のほうが髙かったとしても、ワクチンとの因果関係が証明された事例が見つかったわけですから、自然災害(感染)と人災(ワクチンの副作用)のダブルパンチで人々が苦しめられてきた事実を正しく理解しておく必要があります。
(本記事は、当ホームページあてにお知らせいただいた文献をもとに構成したものです。情報をお寄せくださった方に感謝いたします)
【参考文献】
1) Grady D, et al., Doctor's death after Covid vaccine is being investigated. New York Times, Jan 12, 2021.
2) Ota N, et al., Expression of SARS-CoV-2 spike protein in cerebral arteries: implications for hemorrhagic stroke Post-mRNA vaccination. J Clin Neurosci, Apr 3, 2025.
3) Chui CSL, et al., Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: a self-controlled case series study. Lancet, Jun 25, 2022.
(2025.6.23)
Q&A 病理解剖例から見えてきたワクチン死の真実?
mRNAタイプのコロナワクチンの接種を受けたのち、死亡した人を病理解剖したとの報告例がこれまで多数ありました。しかし、そのほとんどは、通常の病理検査しか行われておらず、たとえばスパイク蛋白との関係まで踏み込んで検討したものはほとんどありませんでした。そのため「因果関係不明」のまま終わってしまっていたのです。
2024年の暮れ、因果関係に一歩、踏み込んだ論文が新たに発表されましたので、着目すべき点をまとめてみました(文献1)。
論文の著者らは、まず2023年5月以前に発表された関係論文を網羅的に集め、PRISMAと呼ばれる国際基準に従って厳選しました。その上で、ワクチン接種日と死亡日との関係が明記されていないものを除外し、さらに3人のベテラン病理専門医が、それぞれ別々にワクチンとの関連性を検証し、2人以上の意見が一致したケースだけを分析対象にしたとのことです。
集まった論文は全部で678編ありましたが、最終的に44の研究で報告された325例のみが信頼に足るケースと判定されました。平均年齢は70.4歳、女性の割合が42.6%でした。ワクチンの種類はさまざまで、もっとも多かったのが41%を占めたファイザー・ビオンテック社製でした。
次のグラフがその結果で、コロナワクチン接種後の日数と死亡者数の関係を表しています(論文で提示されていたグラフをもとに私がイラストとして再構成したもの)。死亡の直接的な原因で多かったのは突然死、心筋梗塞、肺塞栓症、心筋虚血、心筋症・心膜炎、脳出血などでした。特徴的だったのは、ほとんどのケースで複数の臓器に異常が認められたことです。

接種後から死亡までの平均日数は14.3日でしたが、1回目の接種後に限ると7.8日、2回目接種後では23.2日でした。
2024年7月20日付の当ホームページ記事で、ブラッドフォード・ヒルの8条件について解説しました。これは「特定の原因が特定の結果をもたらしているかどうかを検証するための条件」を列挙したものですが、ワクチン接種後、10日内に亡くなった人が82%を占めていた点などもふまえ、8条件はほぼ満たしていると考えてよさそうです。
同じく2024年3月4日付の記事では、日本国内の統計から求めた類似のグラフを提示しましたが、それに比べても格段に説得力あるデータとなっています。この論文の原稿は、いくつかの専門誌に投稿され、掲載が拒否されるなど紆余曲折があったようです(コロナワクチンを否定的に扱っているためと思われます)。
しかし冒頭に紹介したような緻密な配慮がなされており、このテーマで発表されてきた学術論文の中でも、信頼性がもっとも高いというのが私の判断です。
この研究とは別に、米国ミシガン州立大学の研究者は、コロナワクチンが使われ始めた最初の一年間だけで、全米で約29万人が副作用で死亡していたと推測されると報告しています(文献2)。この2つの研究から、コロナワクチンによる死亡は、これまでメディアで語られていた数字をはるかに超える規模で起こっていたことが、あきらかになったように思われます。
【参考文献】
1) Hulscher N, et al., A systematic review of autopsy findings in deaths after COVID-19 vaccination. Science, Public Health Policy, and the Law, Nov, 2024.
2) Skidmore M, COVID-19 illness and vaccination experiences in social circles affect COVID-19 vaccination decisions. Science, Public Health Policy, and the Law, Oct, 2023.
【追補】前回の記事『いまさらそんなことを言われても・・・』について、当ホームページあてに情報提供がありました。CDCの悪口を書いた文献2の著者Martin A Makary氏は、その後、FDAの長官に就任し、現在に至っているとのことです。つまりカンガルー裁判のもう一つの側面は、FDAとCDCの縄張り争いだったということになります。なお前回の記事で引用した2つの文献の両方に、同氏の名前があったことを見落としていましたので、ここに補足させていただきます。
《以前のページへ》
目 次
(目次(青文字)をクリックすると先頭に戻る)
Q0 政府に間違ったデータを提供したのは誰? Q1 ワクチン接種の強制は問題ないのか?
「超過死亡」という言葉にご注意 接種後の副作用で苦しむ人たち
/コロナ致命率の発表値は間違っている /米国における接種義務化と法律事情
/コンピュータ・シミュレーションに騙されるな /接種の強制は倫理的に許されるのか
/地方紙が伝える真実とは
/ワクチン被害の裁判は可能か
/米国の最高裁判決とは
/ワクチン被害の証拠を残そう
Q2 ワクチンを受けない人たちの災難とは? Q3 安心できるワクチンとは?
子育て中の苦悩/アレルギー体質で接種拒否 国産ワクチンを評価する
/悲痛な海外事情 /鼻スプレーワクチン
/打たない人は集団免疫に貢献できないのか
Q4 治療薬はいつできるのか? Q5 なんとか予防はできないのか?
国産初の飲み薬ゾコーバ 薬で予防はできない
/イベルメクチン/抗体カクテル /感染しても重症化しないために
/疑惑の飲み薬モルヌピラビル /肥満がリスクとなる理由が判明
/レムデシビル再評価_有効性に疑問 /自宅療養に備える
/ファイザー社の飲み薬は大丈夫か /アルコール消毒はだめ
/続々登場する新薬のまとめ /意に反して接種してしまったら
/重症化したらこんな病院に行きたい! /民間療法は有効か?
/感染リスクが予測できるアプリ?
/マスクは要らないって本当?
Q6 コロナ禍を終息させる決め手とは? Q7 専門家の言うことは正しいのか?
風船現象を知る データに騙されないための心得帳
/第5波が収束したわけ /運び屋ウイルスのDNA組み込み
/インフルエンザワクチンに学べ /抗体依存性感染増強(ADE)
/インフルエンザワクチンは打つべきか /高齢者の死亡が減少しているわけ
/ワクチンがウイルス変異を助長している /接種を1回で止めてよいか
/インフルとコロナは同時流行するか /後ろ向き調査のまやかし
/全数把握・定点観測って何?
/中和抗体はなぜ無効なのか?
Q8 ウイルスの変異、これからどうなる? Q9 実際に感染したら、どうする?
ウイルスはどのように変異するのか オミクロン株BA.5に感染したら
/変異ウイルスのまとめ /1年後も症状は残るのか
/オミクロン株の種類 /学校の授業は安全か
/オミクロン株はインフルエンザより軽症 /重症化しやすい人の体質:
/デルタミクロンって何 キラーT細胞の仕事とは
/人間の遺伝子は強くなっていく /隔離期間はもっと短くしよう
/悪どいコロナの正体
Q10 ワクチンを巡るデータはねつ造? Q11 うわさのウソ、ホント?
報告されなかったデータ/重症例は増えたか フェイクニュースの元締め
/1回接種で十分/消えた協力者 /基礎疾患のウソ
/ファイザー社の新論文は意味不明 /mRNAは永久に残るか
/むしろ死亡率を高めている証拠 /mRNAは遺伝子に組込まれるか
/消された証人たち/年をとると免疫は? /トゲトゲ蛋白がDNAを破壊するか
/有効期間は2ヵ月/まやかしの有効率
/ワクチン治験を告発した女性
Q12 ワクチンは効いていない? Q13 なぜ医師は正しい知識を持てない?
接種率が高い国ほど感染者は増えている 医師たちが騙されたもう一つの理由
/致命率の計算はほとんど不可能_行政のさじ加減
/接種しても、しなくてもウイルス量は同じ
/繰り返しの接種は大丈夫なのか
/接種完了の施設で集団感染
/ワクチンパスポートに根拠なし
/11歳以下の接種を考える
/オミクロンに中和抗体は無力
/オミクロン用ワクチンは大丈夫?
Q14 なぜ致命的な自己免疫疾患を起こすのか? Q15 因果関係を証明する方法はあるか?
免疫性腎障害/免疫性心臓病 トゲトゲ蛋白はなぜ危険なのか
/免疫性皮膚病/免疫性感染症 /尿中のトゲトゲ蛋白測定に初めて成功
/免疫性眼疾患/ワクチンで突然死 /因果関係を証明する唯一の方法とは
/接種後2ヶ月間で起こること /トゲトゲ蛋白は4ヵ月血中に残る
/接種後半年でわかったこと 【妊娠・出産・育児を考える】
/副作用は脾臓から始まる ワクチンは母乳に影響しないの?
/初めて国内学会で発表された副作用 /妊娠中のワクチン接種は絶対ダメ
/両親の接種は赤ちゃんに影響
/子供と赤ちゃんの接種を考える
Q16 コロナの各検査法の利点と欠点? Q17 あやまちを繰り返さないためには?
PCRの原理を理解しよう 第1回 新型コロナはどこから来たのか
/PCRで困ること/インチキな中和抗体検査 第2回 人々を狂わせたワクチン神話
/3つの検査法の優劣 第3回 メディアのプロパガンダなのか
/抗原検査を練習しておこう 第4回 そろそろ法律家の出番!
/PCR検査を毎週受けた経験談 第5回 新薬とワクチンは期待できるか
/陰性証明は難しい/唾液の検査は確かなの? 第6回 新型コロナはこれからどうなる
第7回 専門家がだまされた統計学とは
第8回 コロナ社会のこれからを考える
Q18 繰り返しのワクチン接種が免疫機能を破壊?
第1回 神様の贈り物を汚すもの(インターフェロン物語)
第2回 改造mRNAに毒性あり(謎のG4構造とは)
第3回 敗者は抹殺せよ!(抗原原罪説の真実)
第4回 mRNAを包む膜に毒性あり(脂質微粒子に猛毒))
第5回 ワクチンを打つと悪性腫瘍に(発がんリスク多数)
第6回 免疫細胞を暴走させるもの(糖鎖に重要なヒント)
第7回 オーバーワクチン症候群(免疫反応が止まる))
第8回 まとめ:ワクチンを打ち続けるのは危険
《執筆者紹介》
現代医療は、世界の巨大医療産業によって操作された偽りのエビデンスによって、間違った方向に誘導されている。その実態を明らかにするため、長年、薬品やがん検診に関するねつ造データの科学的検証を行っている。
著 書
『人はなぜ太るのかー肥満を科学する』(岩波新書)、2006年(11刷)
『がんは8割防げる』(祥伝社新書)、2007年
『ほどほど養生訓』(日本評論社)、2007年(5刷)
『放射能と健康被害 20のエビデンス』(日本評論社)、2011年
『医者の私が、がん検診を受けない9つの理由』(三五館)、2016年(4刷)
『医者が教える「家族に飲ませない薬」』(PHP)、2019年(1刷)
『医療AIの夜明け:AIドクターが医者を超える日』(オーム社)、2019年
『大丈夫か、新型ワクチン』(花伝社)、2021年(2刷)
『本当に大丈夫か、新型ワクチン』(花伝社)、2022年
『新型ワクチン騒動を総括する』(花伝社)、2023年、ほか多数
研究論文
1. Abe T, Okada M, et al., Sleep duration is significantly associated with carotid artery atherosclerosis incidence in a Japanese population. Atheroslcerosis 217: 509-513, 2011.
2. Okada M, et al., Low-density lipoprotein cholesterol can be chemically measured: a new superior metod. J Lab Clin Med 132: 195-201, 2998.
3. Okada M, A metod for clinical data reduction based on "weighted entropy", IEEE Trans Biomed Eng BME-25: 462-467, 1978. など574編
経 歴
京都府舞鶴市生まれ
1972年 新潟大学医学部卒業
1990年 同 医学部教授
2012年 同 名誉教授(国立大学 教授定年退官後の称号)
診 療
高脂血症・高血圧症・糖尿病などの予防治療
超高齢者に特化した医療の模索
受 賞
・新潟日報文化賞、1981年
・臨床病理学研究振興基金「小酒井望賞」、2001年
主な発明・発見・特許
・低密度リポ蛋白中のコレステロールの定量方法(特許3058602)
・超低比重リポ蛋白及び中間比重リポ蛋白のトリグリセライド定量方法(特許4070958)
・LDLコレステロール検査法を世界で最初に開発
・重み付きエントロピー計算法の発明
・Bツリーによる重複情報カウント・アルゴリズムの発見
資 格
・医学博士
・日本循環器学会認定循環器専門医(~2010年)
・日本医師会認定産業医
・AHA Profesional Member(米国心臓学会、上級会員)
・IEEE Senior Member(米国電子工学学会、上級会員)
主な学会・社会活動
・IEEE T-BME(米国電子工学専門誌)共同編集長、1986年
・文部省大学設置・学校法人審議会、専門委員、1997年
・日本エム・イー学会誌『生体医工学』、編集長、1999年
・Frontiers Med Biol Engng(学会誌)、編集長、1999年
・公益信託臨床病理学研究振興基金、審査委員長、2000年
・文部科学省科学研究費補助金、審査委員、2002年
・全国国立大学法人病院検査部会議、議長、2005年
・第32会医療情報学連合大会、大会長、2012年
・Arch Prev Med (米国医学専門誌)、副編集長、2015年
copyright©2021 Masahiko Okada all rights reserved.